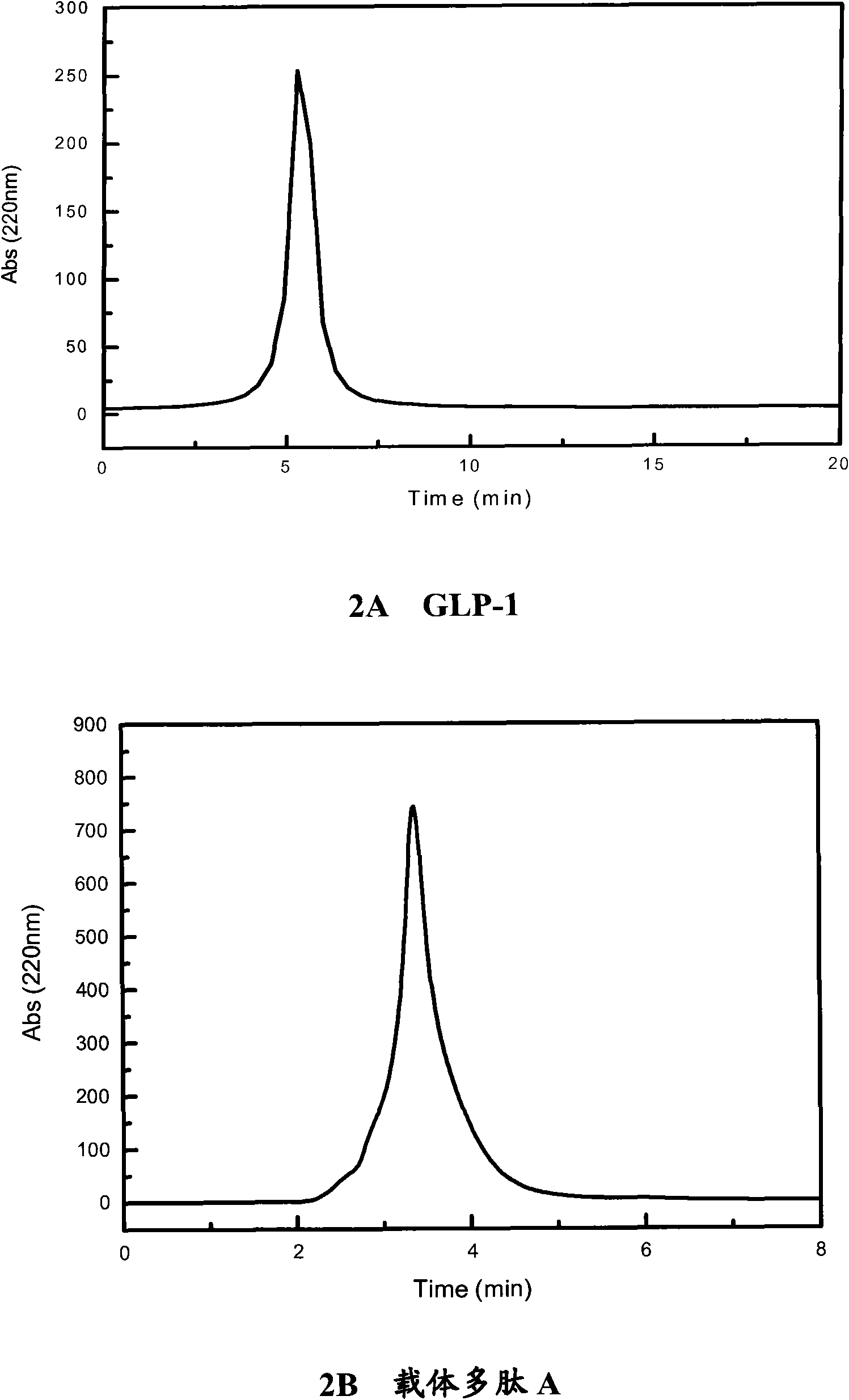
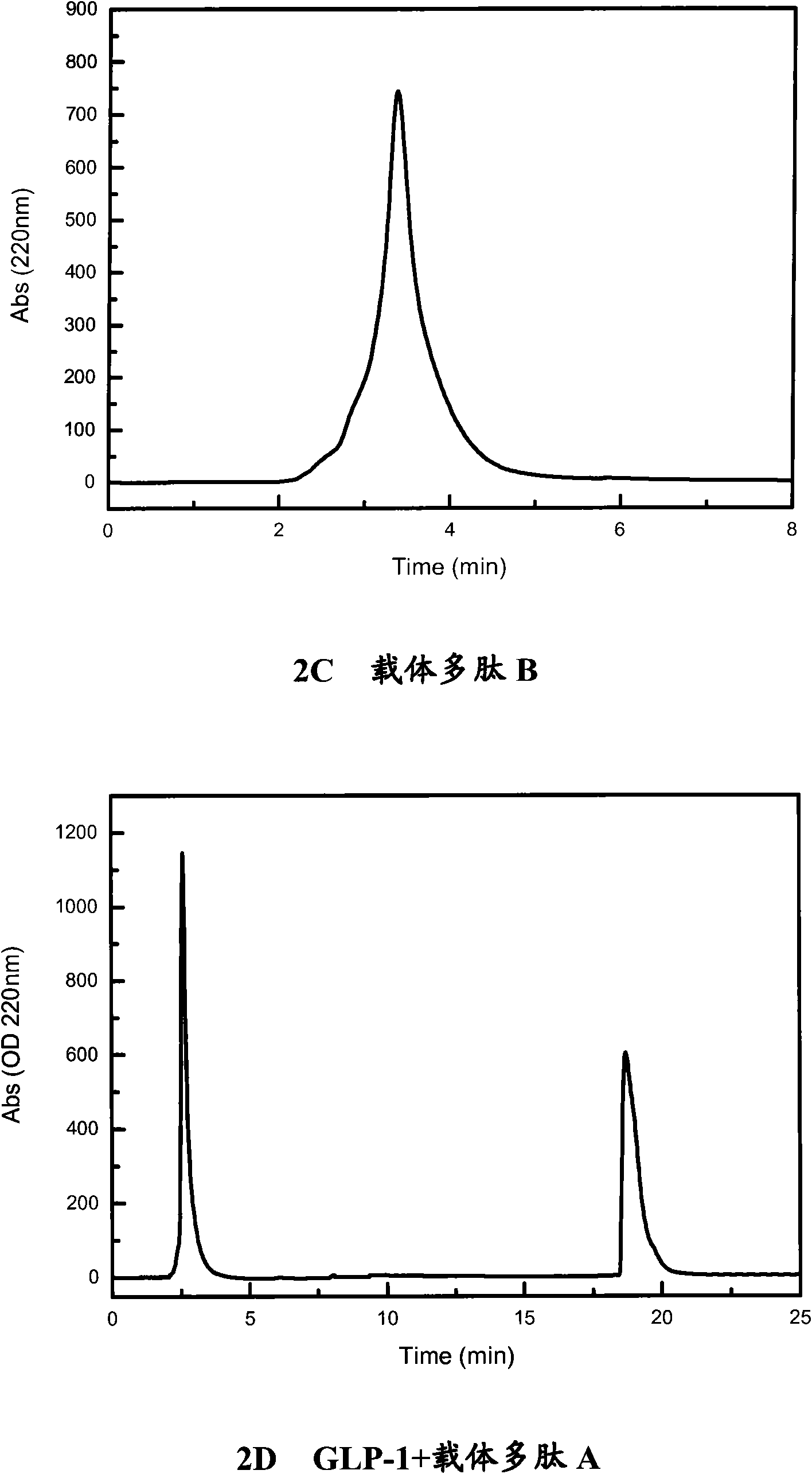
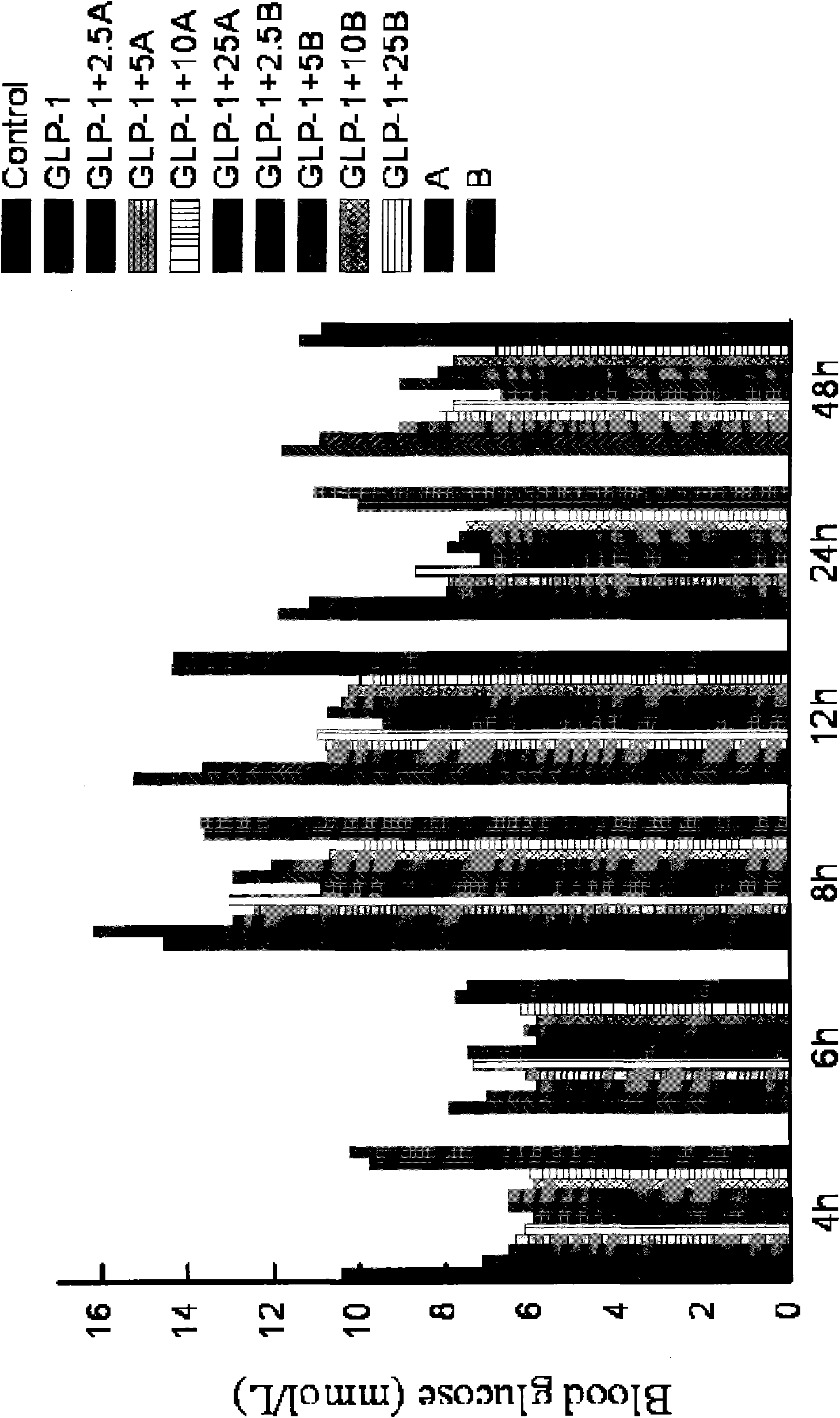Polypeptide complex, pharmaceutical composition, as well as preparation method and application of polypeptide complex
A polypeptide composition and composition technology, applied in the direction of drug combination, pharmaceutical formula, chemical instrument and method, etc., can solve the problems of short half-life of liraglutide, inconvenient clinical use, failure to meet clinical standards, etc., and achieve convenience in clinical practice The effect of promotion and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1: Preparation of carrier polypeptide A
[0056] According to the preferred codon (but not limited to the preferred codon) of Escherichia coli, the base sequence containing polypeptide A is synthesized by the method of total gene synthesis, and the DNA sequence is as follows:
[0057] SEQ ID NO 3:
[0058] 5 TATG ATTGAAGGCCGT GGCCTGTGGTGGAAAGCGTGGTGGAAAGCGTGGTGGAAATCCCTGTGGTGGCGTAAACGTAAACGTAAAGCGTAATAAG 3
[0059] SEQ ID NO 4:
[0060] 5 GATCCTTATTACGCTTTACGTTTACGTTTACGCCACCACAGGGATTTCCACCACGCTTTCCACCACGCTTTCCACCACAGGCC ACGGCCTTCAAT 3
[0061] To bind DNA sequence 3 and sequence 4, add equal molar amounts of SEQ ID NO3 and SEQ ID NO 4 respectively, incubate in a water bath at 95°C for 5 minutes, and then slowly cool down to room temperature. This double-stranded DNA can be connected with the Escherichia coli expression vector pET15b after being digested by NdeI / BamHI. As shown below, the double-stranded DNA synthesized by the above method conta...
Embodiment 2
[0065] Embodiment 2: Preparation of carrier polypeptide B
[0066] According to the preferred codon (but not limited to the preferred codon) of Escherichia coli, the DNA sequence of Polypeptide B synthesized by the method of total gene synthesis is as follows:
[0067] SEQ ID NO 5:
[0068] 5 TATG ATTGAAGGCCGT GGCCTGTGGTGGAAAGTGTGGTGGAAACTGTGGTGGAAAAGCCTGTGGTGGCGCAAACGCCTGCGCAAAGCGTAATAAG 3
[0069] SEQ ID NO 6:
[0070] 5 GATCCTTATTACGCTTTGCGCAGGCGTTTGCGCCACCACAGGCTTTTCCACCACAGTTTCCACCACACTTTTCCACCACAGGCC ACGGCCTTCAAT 3
[0071] To bond DNA sequence 5 and sequence 6, add equal molar amounts of SEQ ID NO3 and SEQ ID NO 4 respectively, place in a water bath at 95°C for 5 minutes, and then slowly cool to room temperature. This double-stranded DNA can be connected with the Escherichia coli expression vector pET15b after being digested by NdeI / BamHI. As shown below, the double-stranded DNA synthesized by the above method contains the sharp ends of 5'NdeI and 3'BamHI,...
Embodiment 3
[0074] Embodiment 3: Preparation and purification technology of recombinant GLP-1
[0075] GLP-1 is a natural product without patent restrictions. In the present invention, biological methods are used to prepare GLP-1, and this patent is also applicable to the use of other polypeptide preparation methods, such as polypeptide chemical synthesis.
[0076] SEQ ID NO 9: HAEGTFTSDVSSYLEGQAAKEFIAWLVKGR
[0077] The coding DNA was synthesized and prepared according to the amino acid sequence of GLP-1 (SEQ ID NO 9). Two complementary DNA single strands were synthesized by Shanghai Sangong, see SEQ ID NO: 7, SEQ ID NO: 8. And referring to the description in Example 1, a double-stranded DNA encoding GLP-1 was prepared, and the double-stranded DNA had an enzyme cutting site (NdeI / BamHI) connected to the vector (pET15b). Referring to the content of Example 1, the expression plasmid of GLP-1 was constructed.
[0078] Recombinant GLP-1 was prepared according to the protein expression, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com