Phosphodiesterase 4d7 as prostate cancer marker
A phosphodiesterase, prostate cancer technology, applied in the determination/inspection of microorganisms, enzymes, hydrolases, etc., can solve problems such as false positives and elevations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
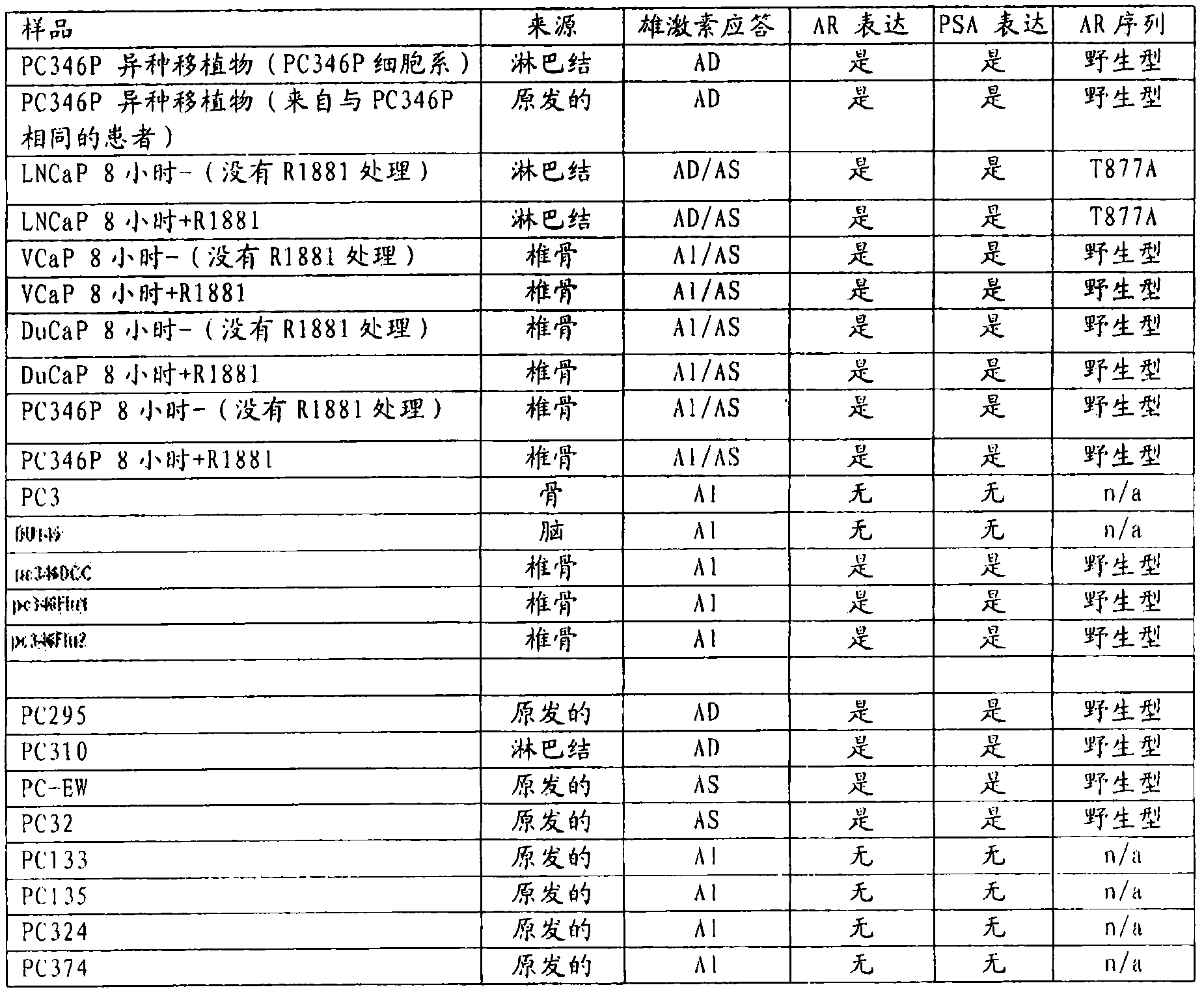
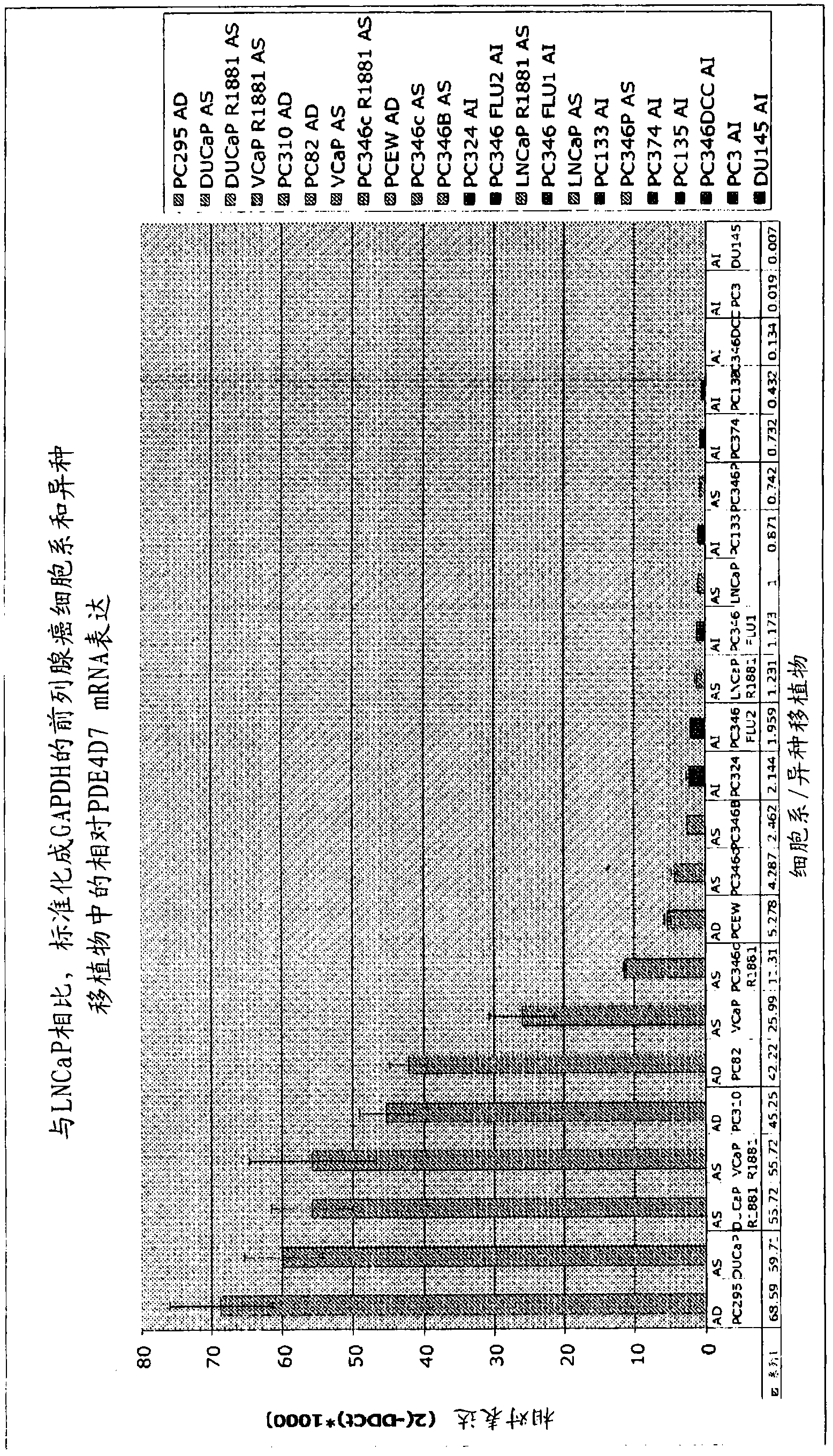
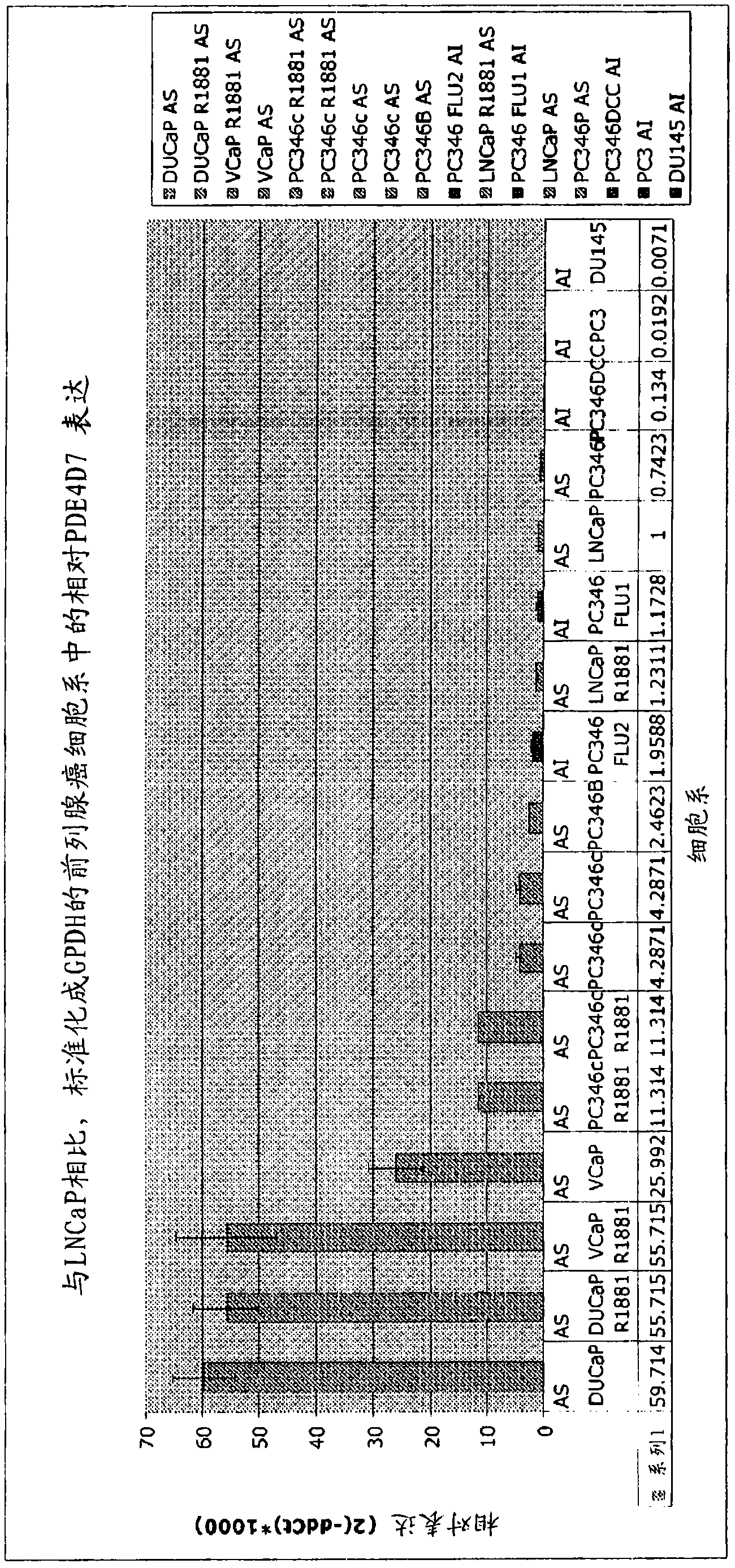
[0344] Example 1 - Quantitative RT-PCR assay of human cell lines and human tissue xenografts
[0345] From figure 1 From the indicated cell lines and human tissue xenografts (see also Marques et al., 2006, Eur. Urol., 49(2):245-57 for additional details), RNA was isolated and transcribed into cDNA by standard procedures . Prepared cDNAs of samples "PC346P xenografts" to "346Flu2" (which are cell lines) and samples "PC295" to "PC374" (which are xenografts) were tested for the expression level of PDE4D7.
[0346] qRT-PCR.: Materials and methods
[0347] Treat RNA samples with DNase to ensure no DNA contamination. RNA samples were treated with DNase I (In Vitrogen) at 37°C for 30 min before cDNA synthesis. 1 μg of RNA samples were then treated with Superscript Vilo (In Vitrogen) following the manufacturer's instructions to synthesize first-strand DNA for qPCR analysis. DNA samples were then treated with RNase H1 at 37°C for 30 min.
[0348] The resulting DNA was diluted t...
Embodiment 2
[0378] Example 2 - Quantitative RT-PCR assay using human tissue samples
[0379] The relative gene expression of human PDE4D7 was evaluated in prostate cancer tissues derived from hormone-sensitive / responsive patients compared with hormone-resistant / castration-resistant patients.
[0380] Materials and methods
[0381] In Table 1 below, details of the samples used in the qPCR measurements of the PDE gene expression experiments are given:
[0382] Table 1: Patient information used in the experiment
[0383]
[0384] All samples were derived from male patients (52-82 years old). The "Tissue" column identifies the tissue that has been removed during the procedure, be it prostate tissue or lymph nodes for staging. The "Type of Tissue" column describes the tissue resection method. If not stated otherwise, this tissue was excised during prostate surgery (prostatectomy). "TURP" is defined as transurethral resection of the prostate. The cDNA pool included 4 samples from pat...
Embodiment 3
[0406] Effect of embodiment 3-PDE4D7 expression on PC3 proliferation
[0407] To test the effect of heterologous expression of human PDE4D7 on the proliferation of androgen-independent cell lines, several gene constructs were transfected into PC3 cells, and the growth of the tested cells was compared to control-treated cell lines. PC3 cells transfected with a dominant-negative form of PDE4D7 in which the catalytic activity of PDE4D7 has been knocked out by genetic mutation of the coding sequence of PDE4D7, as well as PC3 cells transfected with a different isoform of the PDE4D family (i.e. PDE4D 1) PC3 cells, as control untreated PC3 cells.
[0408] In RPMI1640 supplemented with L-glutamine (2mM final concentration) and PenStrep (1%), in 5% CO 2 , 37°C, culture PC3 cells in 5% fetal bovine serum.
[0409] PC3 cells were seeded at a density of 5000 cells per well of a 96-well plate (MTP). Transfection complexes for the genes of interest (PDE4D7, dominant-negative PDE4D7, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com