Injection composition containing oxiracetam and preparation method and application thereof
A technology for injection and composition, which is applied in the field of small-volume injection preparations, can solve the problems of reducing related substances, and achieve the effects of low content of related substances, reducing the possibility of hydrogen bond formation, and simple formula
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
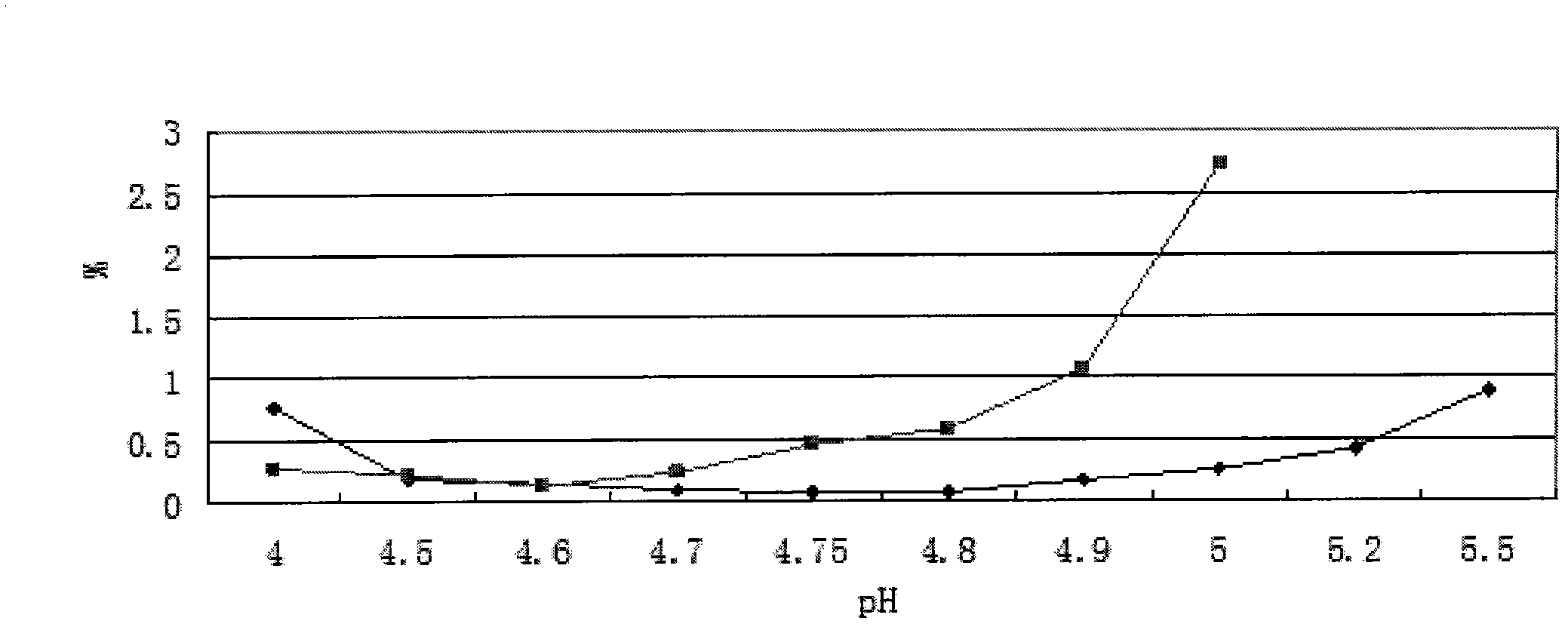
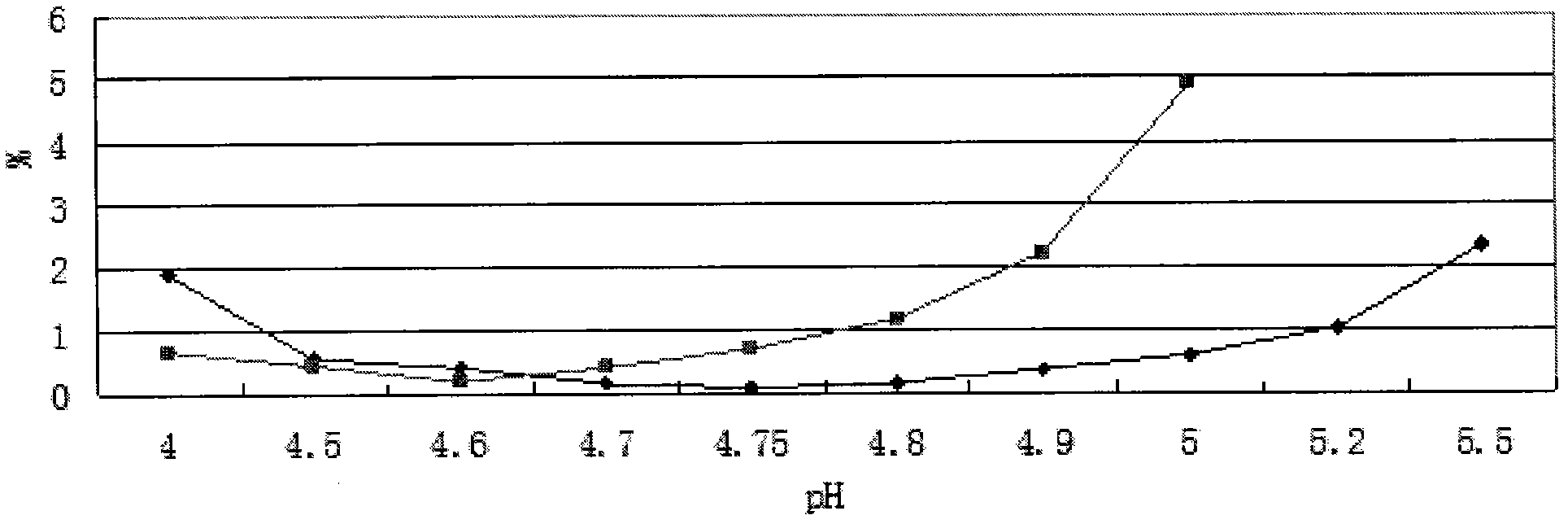
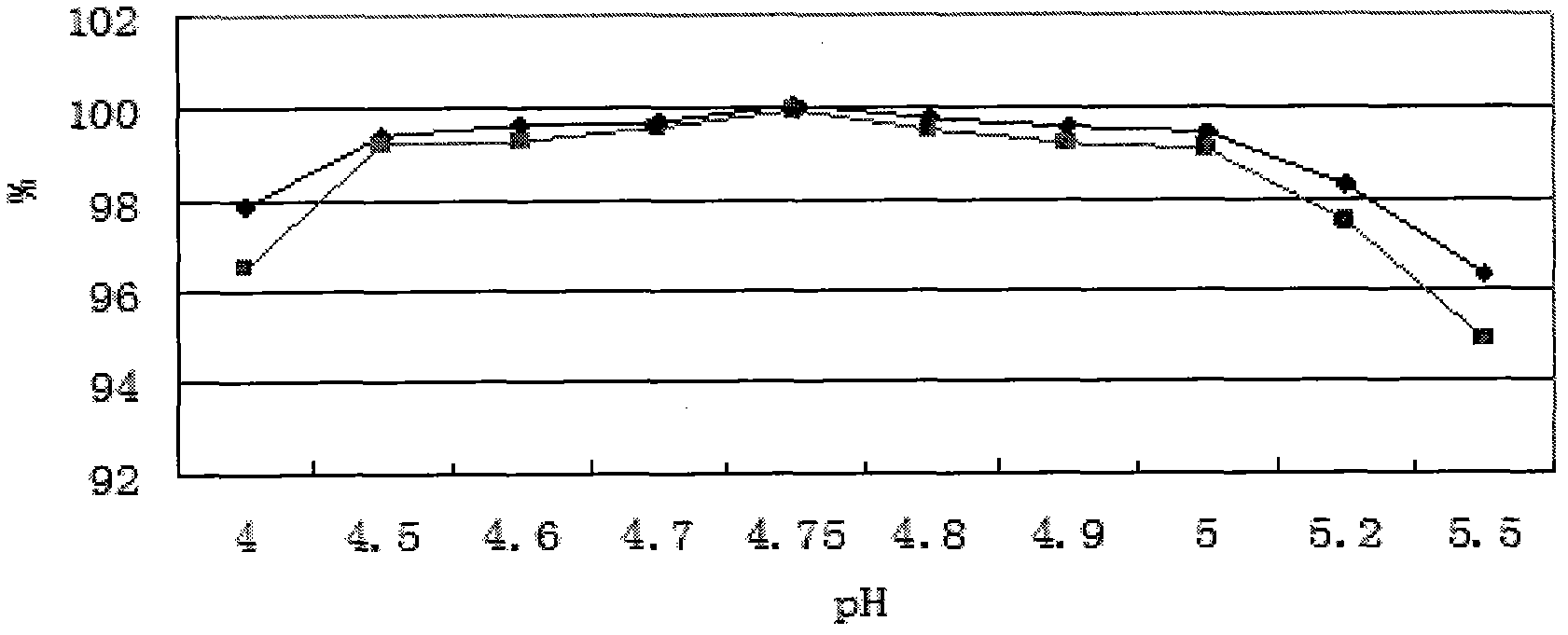
[0051] Add 1kg of oxiracetam to 4L of water for injection, stir to dissolve it; adjust the pH value to 4.8 with 0.5mol / L (ie 0.5N) sodium hydroxide solution; dilute the volume of water for injection to 5L; Microporous membrane filtration, filling filtrate 5ml / bottle, 1g / bottle, melting and sealing; sterilization (temperature: 121°C, constant temperature time: 15 minutes); lamp inspection, ready to use. The preparation should be protected from light, sealed and stored at a storage temperature below 20°C.
[0052] Stability study plan:
[0053] (1) Destructive test: acid, alkali, heat, oxidation test
[0054] (2) Accelerated test: take three batches of pilot test samples of this product, and place them in conditions A and B respectively, A: temperature 40°C±2°C, relative humidity 75%±5% temperature and humidity control box, B: 30 ℃ ± 2 ℃, relative humidity 65% ± 5% in the temperature and humidity control box to take samples regularly, measure the properties, pH, color of the s...
Embodiment 2
[0057] Take 30L water for injection cooled to room temperature, add 10.0kg oxiracetam, stir to dissolve completely; adjust the pH value to 4.7 with 0.51mol / L sodium hydroxide solution; dilute to 50L with water for injection, add 50.0 g needle with activated carbon, stirred and adsorbed for 20 minutes, filtered to remove carbon; filtered the medicinal solution through a 0.22 μm microporous membrane, filled with filtrate, and the filling capacity of each ampoule was 5ml, and sealed; the sample was sterilized (121°C , 15 minutes); lamp inspection, to obtain the injection preparation of oxiracetam.
Embodiment 3
[0059] Add 10.0kg of oxiracetam to 40L of water for injection, stir to dissolve it; adjust the pH value to 4.8 with 0.49mol / L sodium hydroxide solution; adjust the volume of water for injection to 50L; filter the liquid through a 0.22μm microporous filter Membrane filtration, filled with filtrate 5ml / bottle, melted and sealed; sterilized (temperature: 121°C, constant temperature time: 15 minutes); light inspection, ready to use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com