Application of Bacillus licheniformis live bacteria in preparation of medicine for preventing and treating intestinal endotoxemia
A Bacillus licheniformis, endotoxemia technology, applied in antibacterial drugs, pharmaceutical formulations, medical raw materials derived from bacteria, etc. Symptoms, drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
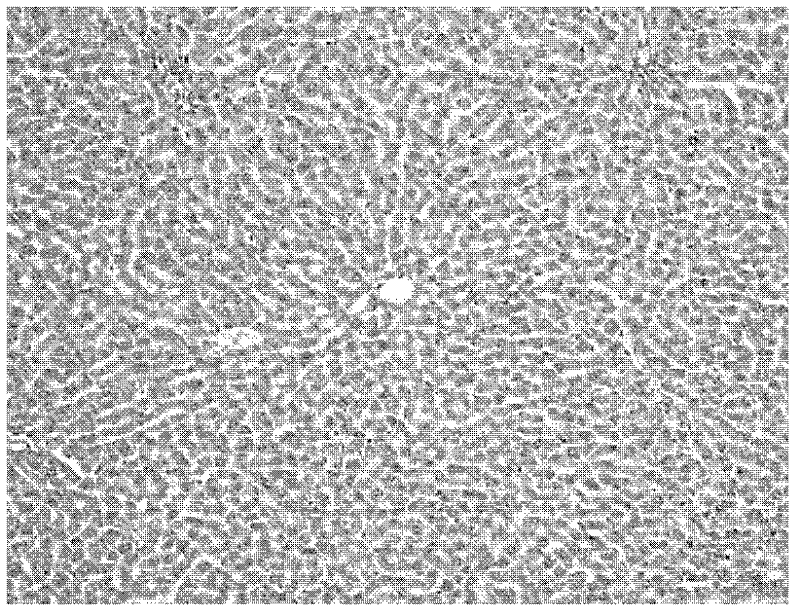
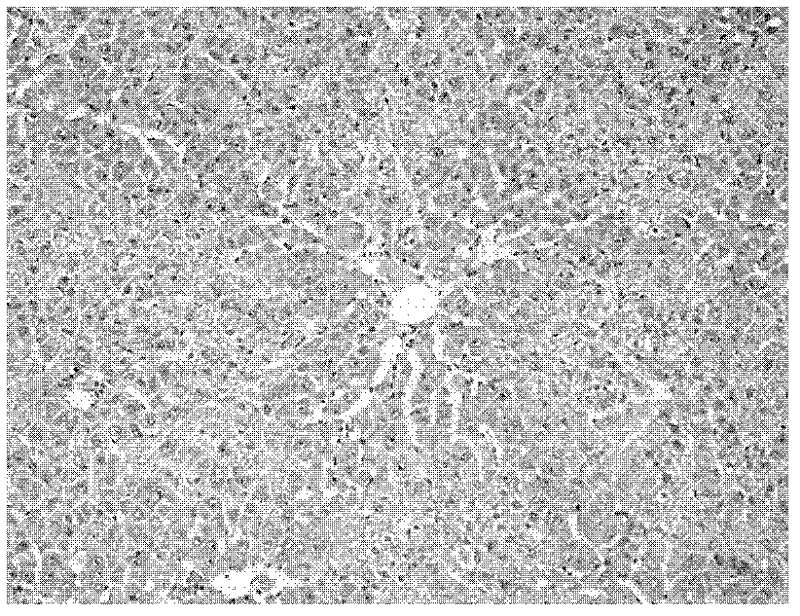
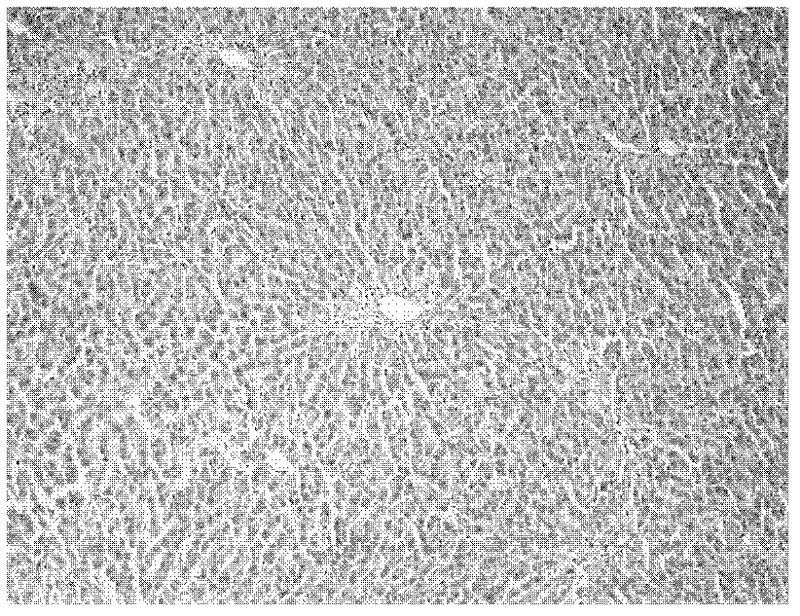
[0036] The invention drug animal experimental scheme is as follows.Animal observation object is the acute acute of sulfuridide (TAA), chronic liver injury of enterotoxin ledchenia caused by mice, detection of toxins, serum galcopanosinase (ALT), and serum gyanosanase (AST)The changes in serum propylene (MDA) and serum lactic acid dehydrogenase (LDH) are analyzed and analyzed liver pathology to observe the role of vectorbacteria live bacteria to prevent and therapeutic effects on enterotoxin.
[0037] There are 160 clean-grade SD rats, male, weighing 240-290g, randomly divided into 3 major groups: 40 acute liver injury prevention control groups, 60 chronic liver injury prevention control groups, and 60 chronic liver injury treatment groups.Each large group is randomly divided into 4 groups: normal control group, TAA model group, Liga Live bacteria live bacteria low -dose group, and high -dose group of Lidobacteria living bacteria.The specific experimental scheme is as follows.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com