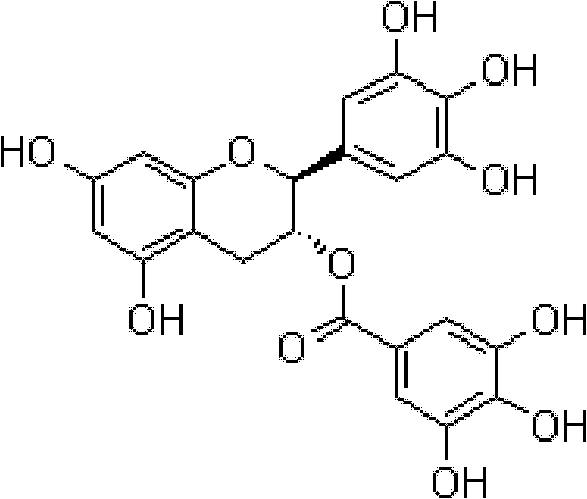
Application of gallocatechin gallate in preparation of benign prostatic hyperplasia resistant medicament
A technology of gallocatechin and gallic acid ester, applied in the direction of drug combination, urinary system diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
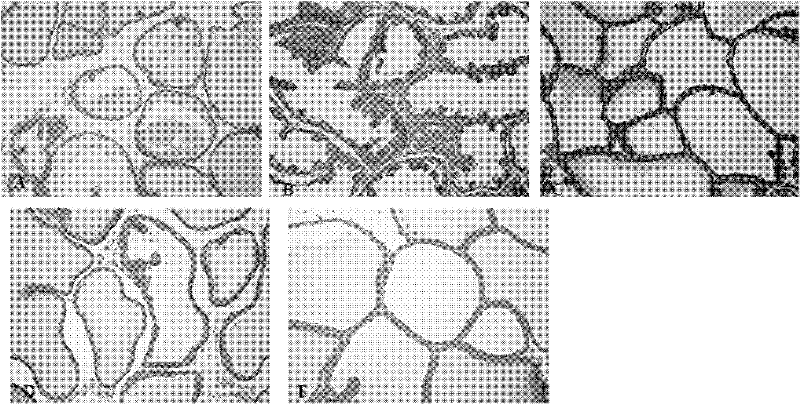
[0021] Example 1 Effect of gallocatechin gallate on rat prostate
[0022] 1. Experimental animals
[0023] Male healthy SD (Sprague-Dawley) rats, clean grade, weighing 250±20g, purchased from Shanghai Xipuer-Bikay Laboratory Animal Co., Ltd., certificate number: 00800160568, production license number: SCXK (Shanghai) 2008-0016 .
[0024] 2. Modeling, grouping and administration
[0025] The above-mentioned 35 rats were generally fed for one week to adapt to the environment. After ordinary observation showed no abnormalities, 7 animals were randomly selected as the normal control group, and the remaining 28 animals were used for modeling. After being anesthetized with ether, the skin was routinely disinfected under aseptic conditions, the bilateral testes were removed through the scrotum, the stump was ligated to ensure hemostasis, the skin was sutured, and a certain amount of ofloxacin injection was dripped to prevent infection. Intramuscular injection (im) penicillin 400,...
Embodiment 2
[0058] The preparation of embodiment 2 gallocatechin gallate liposomes
[0059] Dissolve lecithin, cholesterol and gallocatechin gallate in absolute ethanol and chloroform (the volume ratio of absolute ethanol and chloroform is 1: 1, and the total volume is 15ml) in a ratio of 3g: 1g: 0.1g by mass. Add 2 mg of vitamin E to the mixed solution to obtain solution A, dissolve 33 mg of folic acid in 330 μl dimethyl sulfoxide to obtain solution B, mix solutions A and B and add 9 g of lactose to obtain mixture C, remove the solvent from solution C to obtain Phospholipid film; the obtained phospholipid film is dissolved in water, liposomes are obtained after hydration, the liposomes are filtered through a 0.2 μm filter membrane, and the liposome solution is gradually mixed in a high-pressure homogenizer under a pressure of 200-400Mpa Step pressurized and homogenized for 2 hours, the obtained liposome was a clear and uniform solution. The obtained liposome solution was freeze-dried to...
Embodiment 3
[0061] The preparation of embodiment 3 gallocatechin gallate capsules
[0062] Prescription: 500 g of gallocatechin gallate, 15 g of starch, 5 g of magnesium stearate (about 0.5%), made into 1000 capsules.
[0063] Preparation: Pass gallocatechin gallate, starch and magnesium stearate through an 80-mesh sieve, mix them evenly, and put them into empty capsules to obtain the product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com