Method for preparing low-silicon Na-A/X cocrystallization zeolite in pure sodium aluminosilicate system
A pure sodium aluminosilicate and co-crystallization technology, which is applied in the directions of crystalline aluminosilicate zeolite, A-type crystalline aluminosilicate zeolite, fauhhedral crystalline aluminosilicate zeolite, etc., can solve the problem of low exchange capacity and X-type zeolite. The problem of high silicon-aluminum ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Main raw materials and reagents used: water glass (wherein the content of active ingredient is SiO 2 26.79wt%, Na 2 O 10.62wt%), sodium aluminate (wherein the content of active ingredient is Al 2o 3 43.3 wt%, Na 2 O 40.35wt%), sodium hydroxide (analytical grade, purity 96wt%).
[0022] A. Synthesis of activated silicon source:
[0023] Weigh 6.42 g of NaAlO 2 Dissolve in 85.16 g of deionized water and stir for 10 minutes until clear. Add 18.18 g of solid NaOH and stir for 10 minutes to prepare a perbasic sodium aluminate solution, which will be used when the temperature drops to room temperature. Weigh 24.00 grams of water glass, add 24.49 grams of the above prepared peralkali sodium aluminate solution dropwise into the weighed water glass, stir while adding, and then stir for 10 minutes after adding. Sealed and static aged at room temperature (25° C.) for 24 hours to prepare an activated silicon source. The molar ratio of the final reaction mixture is: NaO 2...
Embodiment 2
[0028] The materials and reagents used are the same as in Example 1; the preparation and aging method of the activated silicon source are the same as in Example 1.
[0029] Weigh out 1.25 g of NaAlO 2 Dissolve in 27.18 g of deionized water, stir for 10 minutes to dissolve, add 0.33 g of solid NaOH, stir to dissolve, and make a low-alkali sodium aluminate solution. Weigh 6.29 g of the above-prepared activated silicon source, slowly add the prepared low-alkali sodium aluminate solution into the weighed activated silicon source, stir while adding, and then stir for 30 minutes after the addition. The reaction mixture was poured into a reaction kettle for crystallization, and the crystallization condition was 90° C. for 6 hours. The molar ratio of the reaction mixture is: NaO 2 :Al 2 o 3 : SiO 2 :H 2 O=4.0:1:2.5:300.
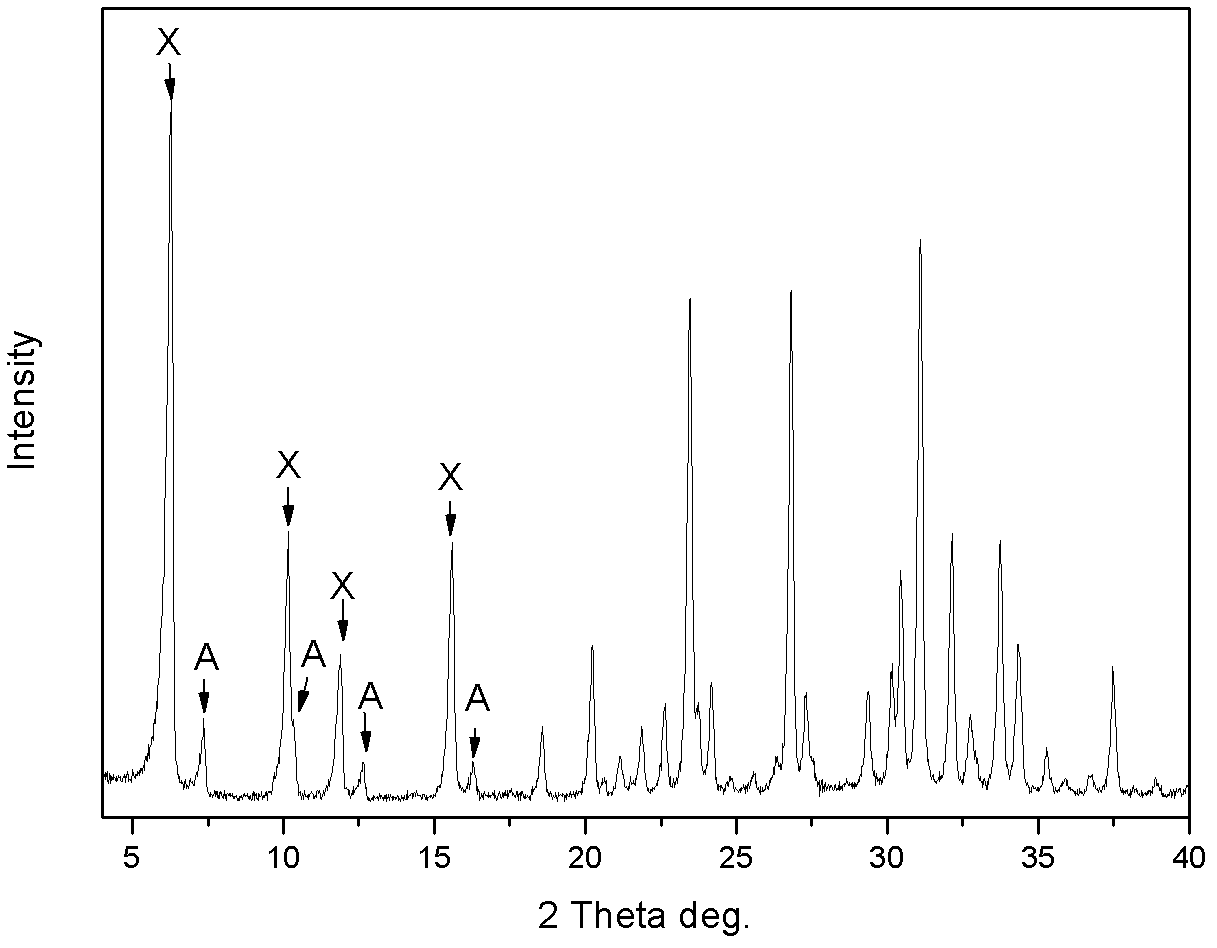
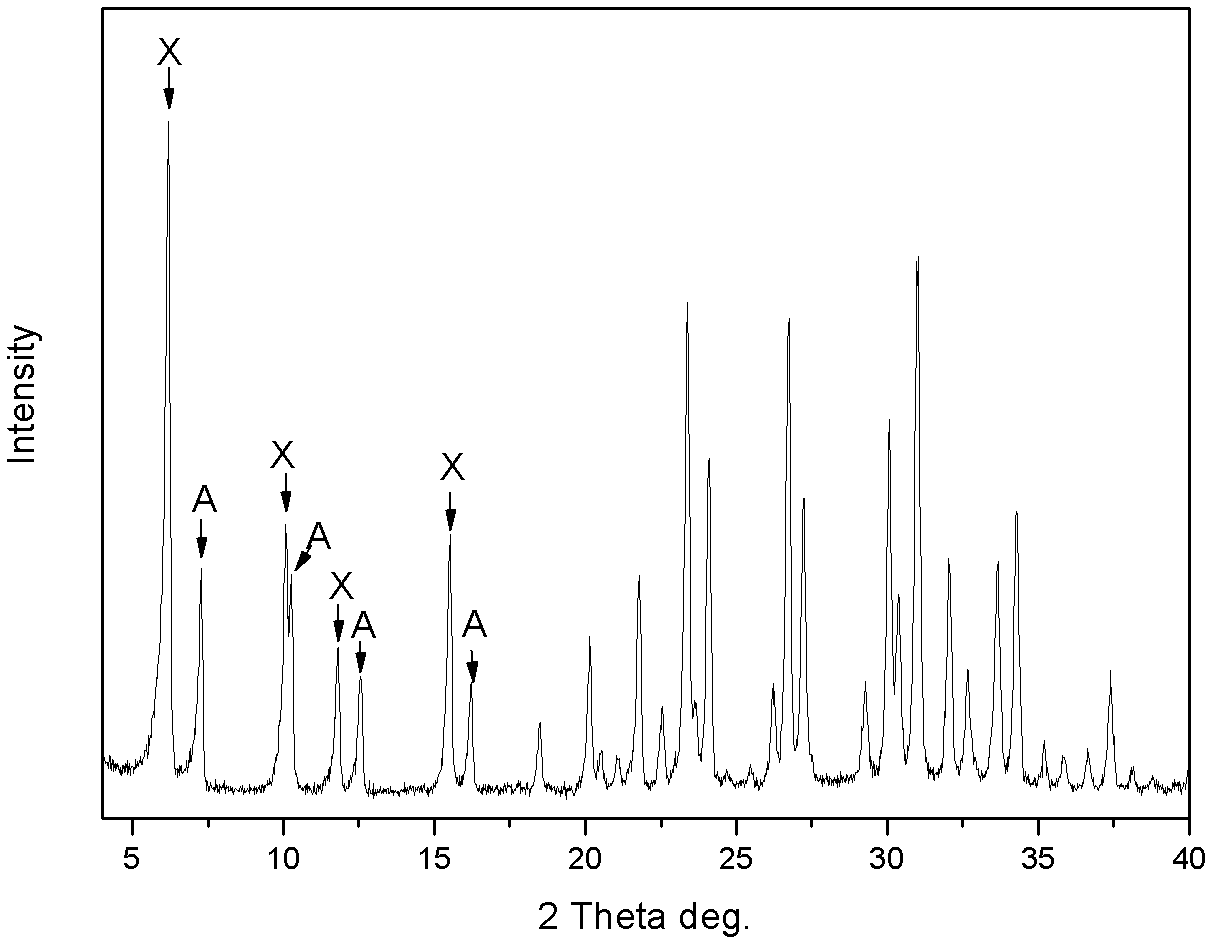
[0030] The product was analyzed by X-ray diffraction to be Na-A / X co-crystallized zeolite, wherein the proportion of A-type zeolite was 38wt%, and X-type zeol...
Embodiment 3
[0032] The materials and reagents used are the same as in Example 1; the preparation and aging method of the activated silicon source are the same as in Example 1.
[0033] Weigh 5.81 g of NaAlO 2 Dissolve in 127.8 g of deionized water, stir for 10 minutes to dissolve, add 1.54 g of solid NaOH, stir to dissolve, and make a low-alkali sodium aluminate solution. Weigh 31.70 g of the above-prepared activated silicon source, slowly add the prepared low-alkali sodium aluminate solution into the weighed activated silicon source, stir while adding, and then stir for 30 minutes after the addition. The reaction mixture was poured into a reaction kettle for crystallization, and the crystallization condition was 10 hours at a temperature of 75°C. The molar ratio of the reaction mixture is: NaO 2 :Al 2 o 3 : SiO 2 :H 2 O=4.4:1:2.5:300.
[0034] The product was analyzed by X-ray diffraction to be Na-A / X co-crystallized zeolite, wherein the proportion of A-type zeolite was 14wt%, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com