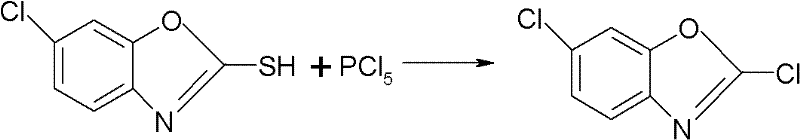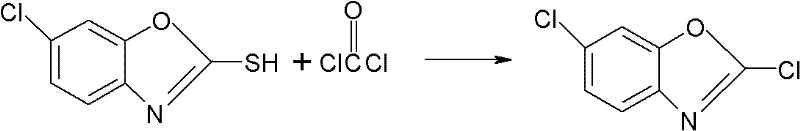Synthesizing method for preparing high-purity 2,6-dichloro benzoxazole
A technology of dichlorobenzoxazole and chlorobenzoxazole, which is applied in the field of pesticide synthesis, can solve the problems of generating a large amount of phosgene, easily generating flushing materials, and dangerous operation, achieves mild reaction conditions and process, and simplifies the difficulty of operation. , the effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Drop into 50 grams (percent) 2-mercapto-6-chlorobenzoxazole and 250ml toluene and 30 grams (percentage) of bis(trichloromethyl) carbonate in 500ml four-necked reaction bottle, stir and heat up to 50 ℃, start to heat up at a rate of 0.5 ℃ / min, and keep warm for 10 minutes every time the temperature rises by 10 ℃. Hours, after the heat preservation is over, remove the solvent by distillation under reduced pressure, keep a vacuum of -0.07MPa during distillation, steam 200ml of toluene, cool to 0°C, stir for 0.5 hours, and centrifuge at a high speed to obtain 2,6-dichlorobenzoxazole, weight 43.5 grams, with a mass content of 99.2%, the recovery of the mother liquor is applied to the synthesis of the next batch of materials, and the molar yield is 98.1%. mp: 48~49℃.
[0034] Data:IR(KBr):v3271.4,3105.9,3036.1,1880.2,1781.5,1731.7,1613.8,1602.2,1528.8,1479.5,1455.8,1422.9,1392.4,1340.3,1322.4,1303.4,1259.7,1242.5,1218.3,1170.4,1143.7 , 1114.3, 1110.5, 1050.8, 1026.9, 941.2,...
Embodiment 2
[0036] In the 500ml four-necked reaction flask, drop into 50 grams (percentage) 2-mercapto-6-chlorobenzoxazole and 250ml xylene and 30 grams (percentage) of bis(trichloromethyl) carbonate, stir and heat up to 50°C, start to heat up at a rate of 0.5°C / min, and keep warm for 10 minutes every time the temperature rises by 10°C. When the temperature rises to 110°C, keep warm for 1 hour, then add 1.19 grams of trichloromethyl chloroformate dropwise, and keep warm for 0.5~ 1 hour, after the heat preservation is over, remove the solvent by distillation under reduced pressure, keep a vacuum of -0.07MPa during distillation, distill 200ml of xylene, cool to 0°C, stir for 0.5 hours, and centrifuge at high speed to obtain 2,6-dichlorobenzoxazole , a weight of 42.1 grams, a mass content of 99.0%, the recovery of the mother liquor is applied to the synthesis of the next batch of materials, and the molar yield is 98.1%.
Embodiment 3
[0038]Drop into 50 grams (percent) 2-mercapto-6-chlorobenzoxazole and 250ml toluene and 35 grams (percentage) of bis(trichloromethyl) carbonate in 500ml four-necked reaction flask, stir and heat up to 50 ℃, start to heat up at a rate of 0.5°C / min, and keep warm for 10 minutes every time the temperature rises by 10°C. Hours, after the heat preservation is over, remove the solvent by distillation under reduced pressure, keep a vacuum of -0.07MPa during distillation, steam 200ml of toluene, cool to 0°C, stir for 0.5 hours, and centrifuge at a high speed to obtain 2,6-dichlorobenzoxazole, weight 47.5 grams, with a mass content of 99.5%, the recovery of the mother liquor is applied to the synthesis of the next batch of materials, and the molar yield is 98.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com