Thermostable mutant of pyrroloquiniline quinine-dependent glucose dehydrogenase and high-throughput screening method thereof
A thermal stability and mutant technology, applied in the field of genetic engineering of enzymes, can solve problems such as poor thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
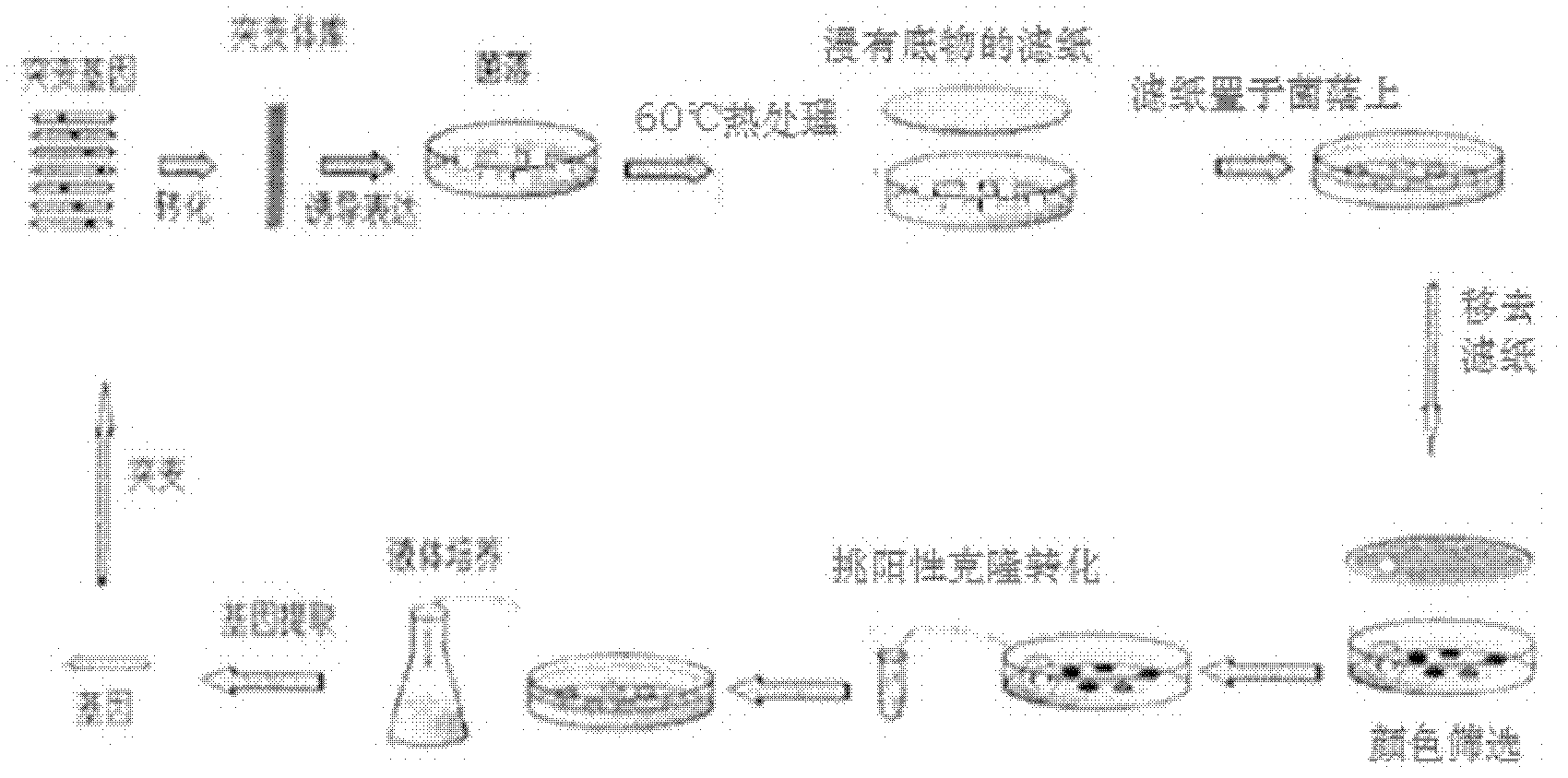
Examples
Embodiment Construction
[0018] Two distinct types of quinone proteases with glucose dehydrogenase activity (membrane-bound and soluble) are grouped together under EC 1.1.5.2, but these two types of enzymes are not related.
[0019] For the present invention only the soluble glucose dehydrogenase s-GDH is relevant, improved variants of which are discussed below.
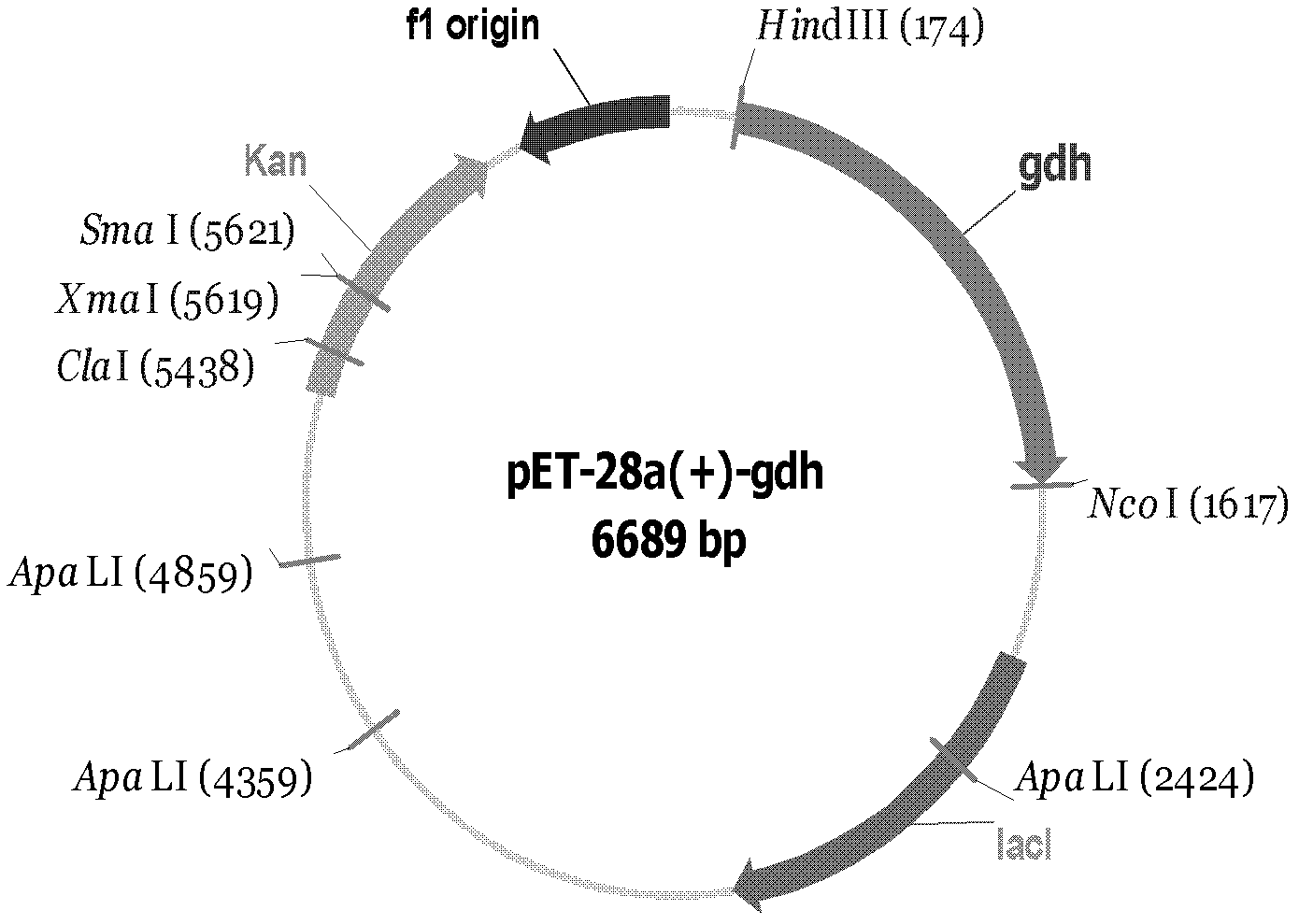
[0020] It is known in the art that the wild-type DNA sequence of s-GDH can be isolated from strains of Acinetobacter. Most preferentially was isolated from Acinetobacter calcoaceticus strain LMD79.41. The DNA sequence and polypeptide sequence of the wild-type s-GDH are given in SEQ ID NO: 1 and SEQ ID NO: 2, respectively.
[0021] The recombinant vector of the present invention is an expression vector, and appropriate vectors can be used according to the needs of expression and purification, such as pET series, pQE series, pMAL series, pGEX series and other expression vectors suitable for E. Expression vectors for other prokaryotic cells, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com