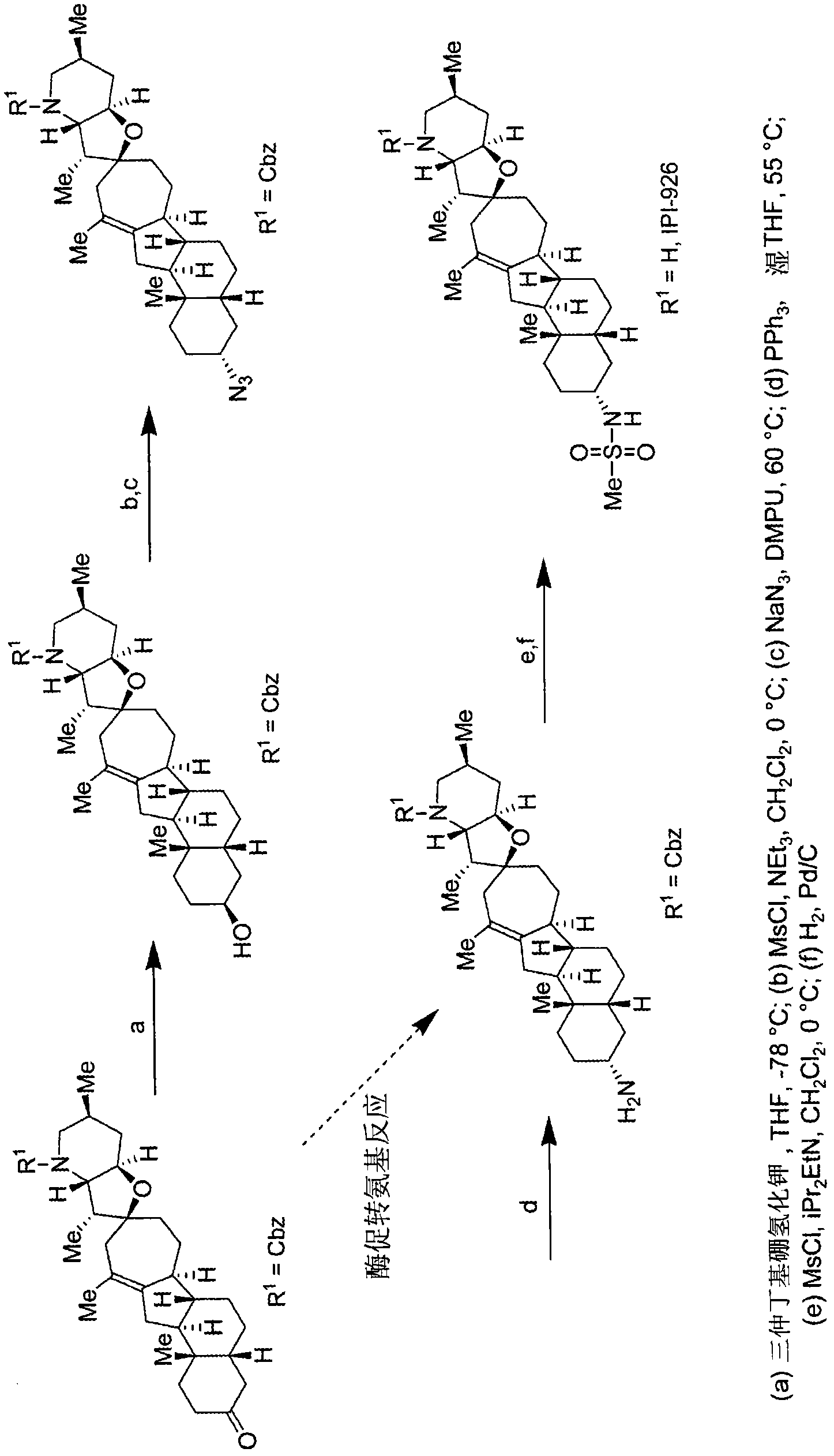
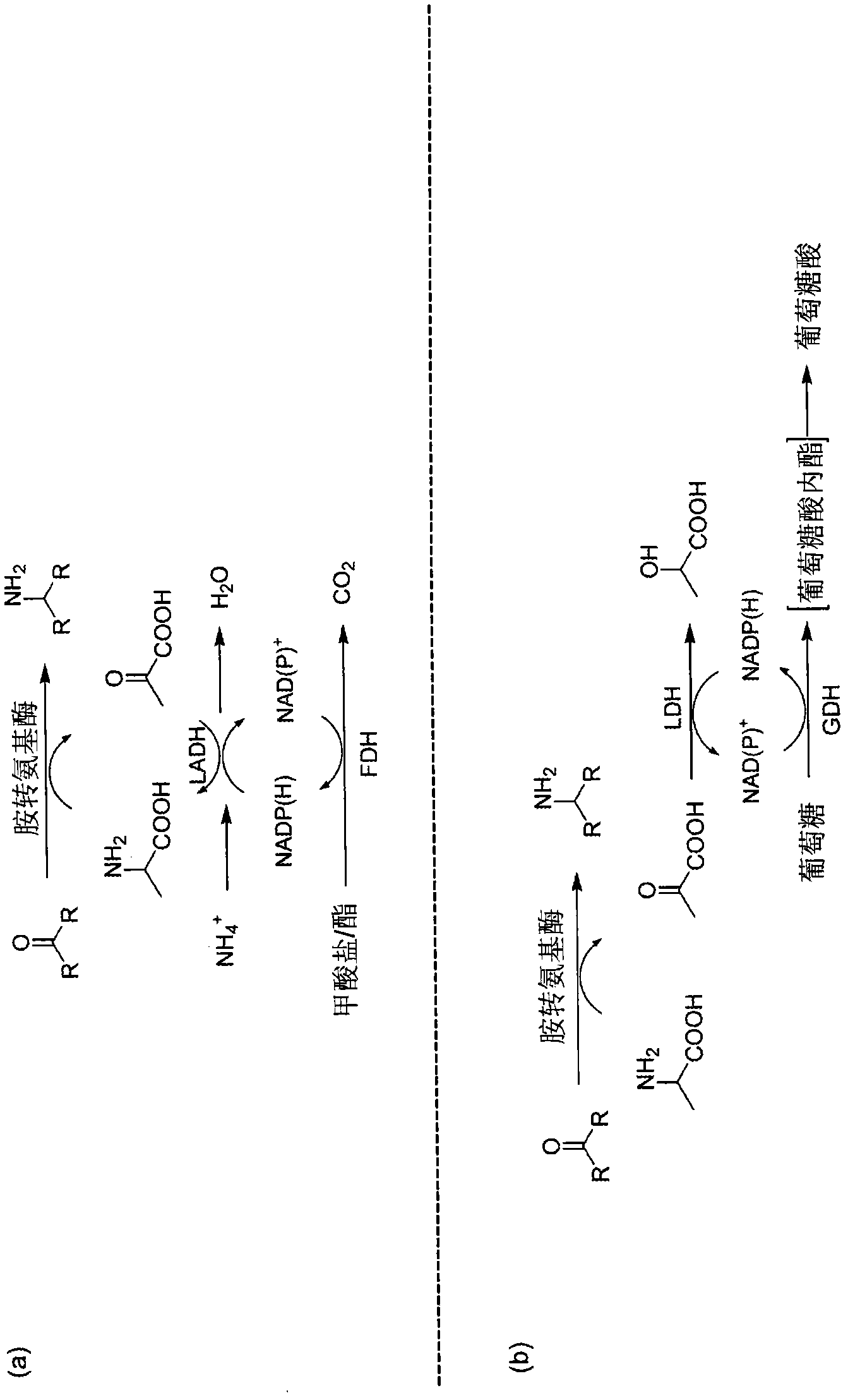
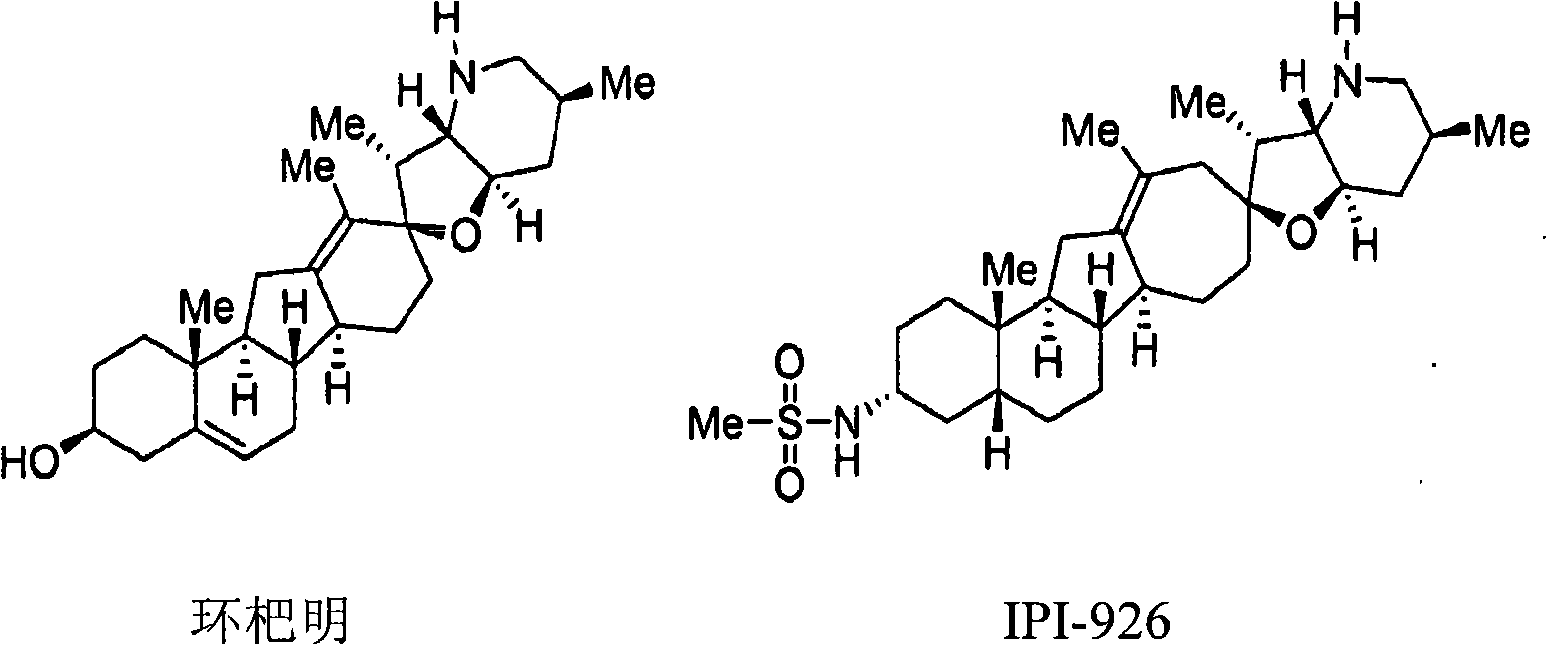
Enzymatic transamination of cyclopamine analogs
A cycloalkyl and aminoase technology, applied in organic chemistry, fermentation, etc., can solve the problems of low solubility and weak acid sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0304] In some embodiments, a method for preparing a compound of formula (R)-(II-a) or a salt thereof from a compound of formula (I-a) or a salt thereof is provided:
[0305]
[0306] in:
[0307] R 1 is H, aralkyl or -CO 2 R 16 ;
[0308] R 16 is H, alkyl, alkenyl, alkynyl, aryl, cycloalkyl, heterocycloalkyl, aralkyl, heteroaryl, heteroaralkyl or -[C(R 20 ) 2 ] p -R 21 , where p is 0-6;
[0309] R 20 is H, alkyl, alkenyl, alkynyl, aryl, cycloalkyl, heterocycloalkyl, aralkyl, heteroaryl, or heteroaralkyl; or any two R's present on the same substituent 20 together form an optionally substituted 4-8 membered ring;
[0310] R 21 for-OR 22 , -N(R 22 )C(=O)R 22 , -N(R 22 )C(=O)OR 22 , -N(R 22 ) SO 2 (R 22 ), -C(=O)R 22 N(R 22 ) 2 , -OC(=O)R 22 N(R 22 )(R 22 ), -SO 2 N(R 22 )(R 22 ), -N(R 22 )(R 22 ), -C(=O)OR 22 , -C(=O)N(OH)(R 22 ), -OS(O) 2 OR 22 ,-S(O) 2 OR 22 ,-OP(=O)(OR 22 )(OR 22 ), -N(R 22 )P(O)(OR 22 )(OR 22 ) or -P(=O)(OR 22 )(...
Embodiment
[0355] Having generally described the present invention, it will be more readily understood by reference to the following examples, which are given by way of illustration only and are not intended to limit the invention.
[0356] Enzymatic transamination reaction of compound of formula (I-a)
[0357]
[0358] Materials and methods
[0359] Enzymes. Amine transaminases were purchased from commercial sources, stored at -20°C, and used as received: ATA-113 (Caudex, Redwood City, Canada; Lot No. 104020902); ATA-117 (Caudex, Inc., Redwood City, Canada; Lot No. 104020902); Omega-transaminase from Vibrio riverina (Fluka; Cat. No. 08374); Glutamate-pyruvate transaminase (Fluka); Spectrum transaminase (Phloka).
[0360] Coenzymes. Coenzymes used during the study included: L-alanine dehydrogenase (LADH, Sigma, no. A7653-100U), formate dehydrogenase (FDH, Caudix, FDH -101) and pyruvate reductase mixture (PRM-102, Caudix), which is lactate dehydrogenase (LDH), glucose dehydrogenas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com