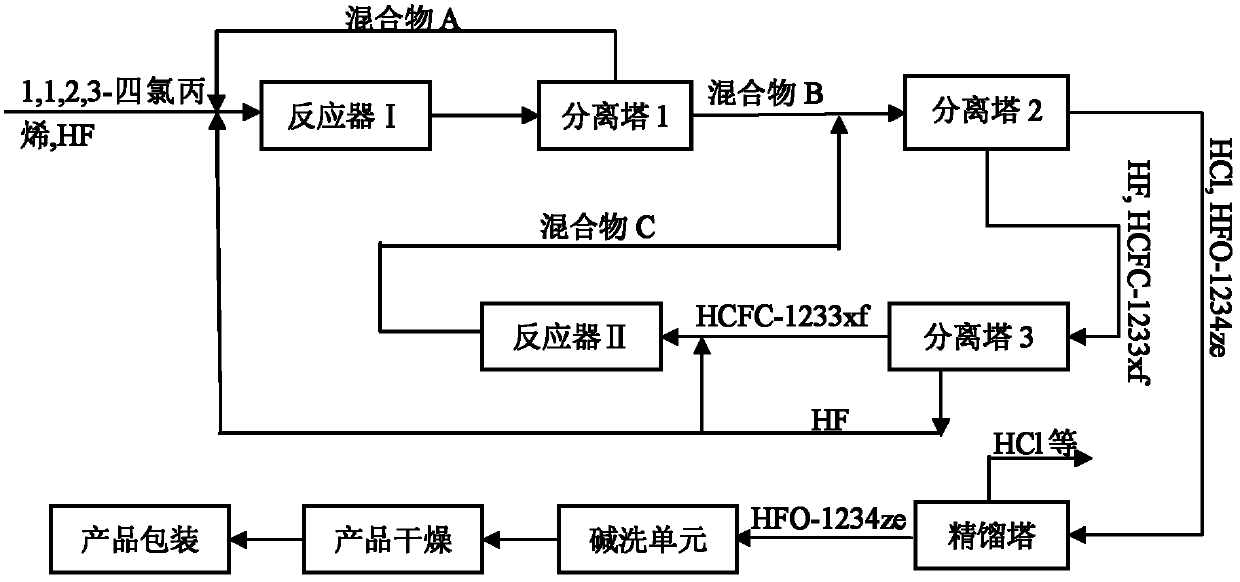
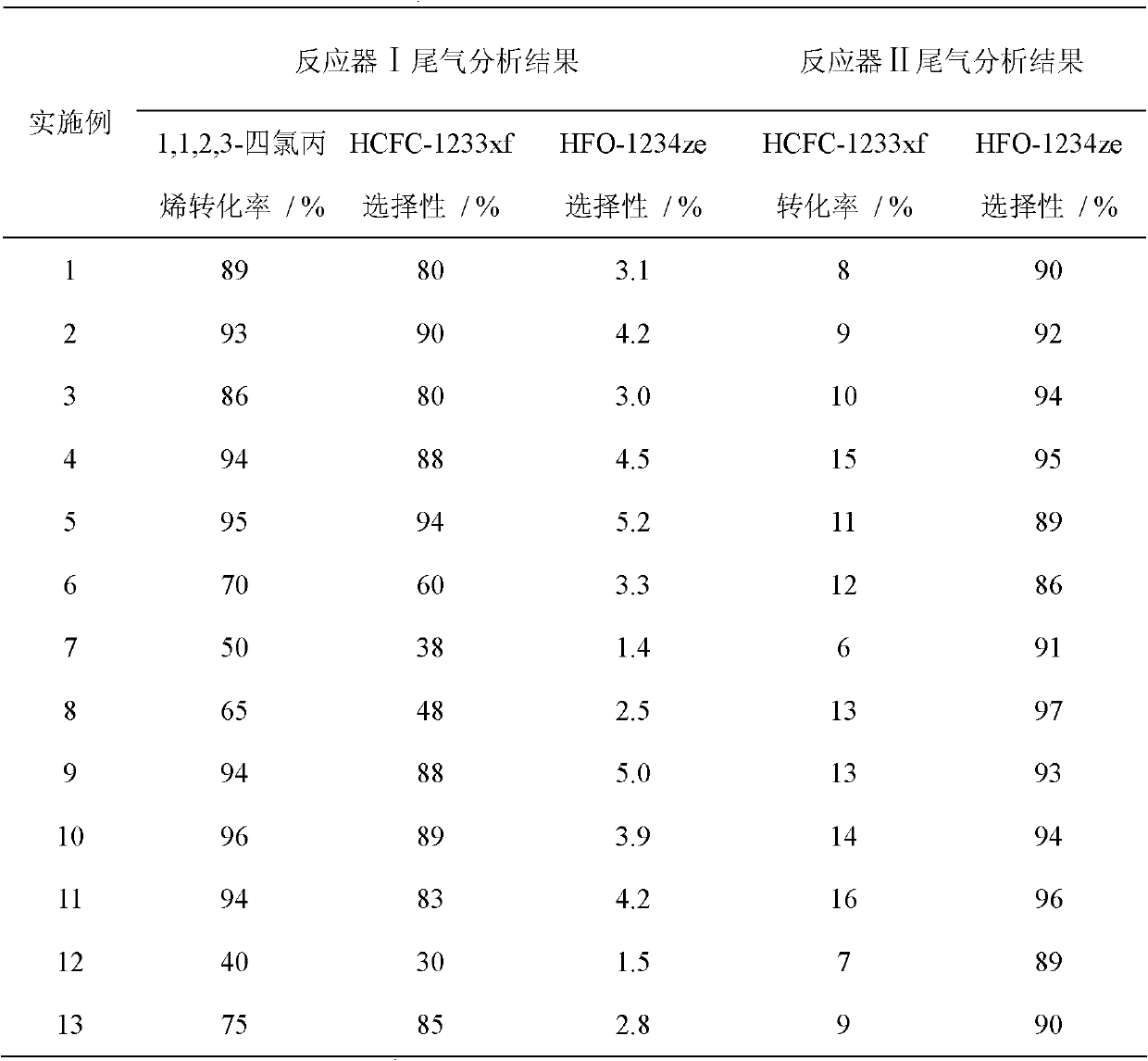
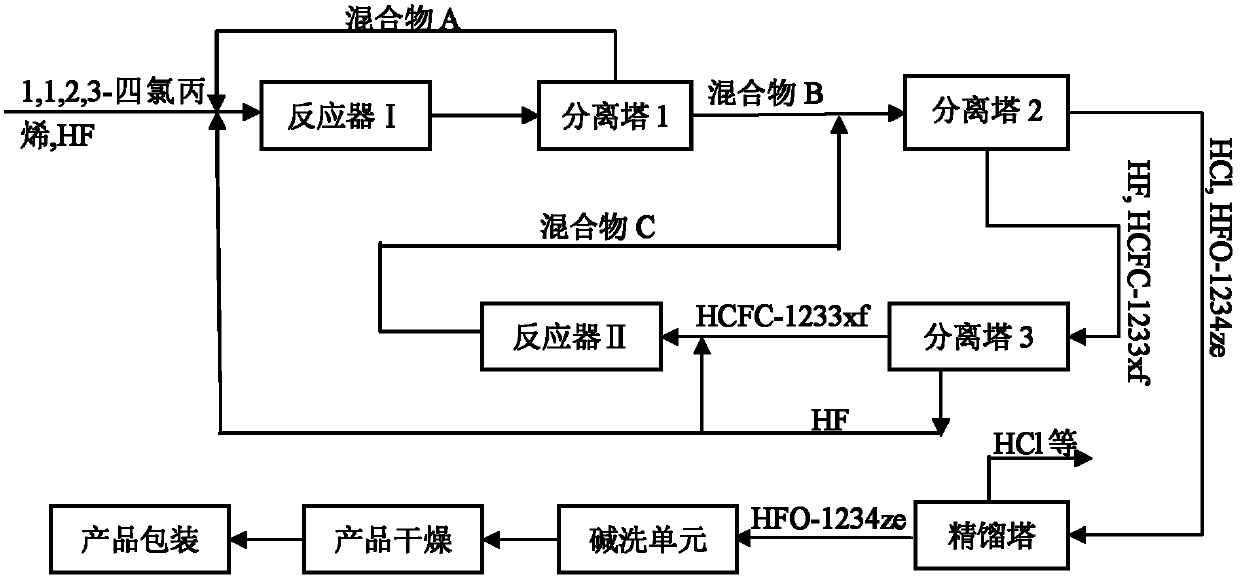
Preparation method of 1,1,1,3-tetrafluoropropene
A technology of tetrafluoropropene and tetrachloropropene, which is applied in 1 field, can solve the problems of complex process, poor economy, difficulty in obtaining HCFC-1233zd and HFC-245fa, etc., and achieve the effect of simple process route and easy price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Catalyst A preparation. First, weigh Al according to the molar ratio of Cr to Al of 9:1 2 o 3 ·H 2 O and Cr(NO 3 ) 3 9H 2 O, mix evenly with an appropriate amount of water, add a certain amount of ammonium carbonate until the precipitation is complete, then separate and wash until the filtrate is neutral, and then dry the precipitate at 120°C. Shape the dried precipitate at 500°C, N 2 Calcined in the atmosphere for 10 hours to obtain a catalyst precursor; the catalyst precursor was treated with HF at 350° C. for 5 hours to obtain catalyst A.
[0034] Catalyst B, according to AlF 3 Loading capacity 1% (activated carbon mass fraction), weigh Al(NO 3 ) 3 9H 2 O and activated carbon, mixed with appropriate amount of water, dried at 400°C N 2 Roasting in the atmosphere for 5 hours; then according to the Pd loading 0.2% (activated carbon mass fraction), measure H 2 PdCl 4 The solution was mixed with the above-mentioned calcined product, impregnated overnight, and...
Embodiment 2
[0038] Catalyst A preparation. First, weigh Al according to the molar ratio of Cr to Al of 8:2 2 o 3 ·H 2 O and Cr(NO 3 ) 3 9H 2 O, mix evenly with an appropriate amount of water, add a certain amount of ammonium carbonate until the precipitation is complete, then separate and wash until the filtrate is neutral, and then dry the precipitate at 120°C. The dried precipitate was shaped and calcined at 500°C in N2 atmosphere for 10 hours to obtain a catalyst precursor; the catalyst precursor was treated with HF at 350°C for 5 hours to obtain catalyst A.
[0039] Catalyst B, according to AlF 3 Loading capacity 1% (activated carbon mass fraction), weigh Al(NO 3 ) 3 9H 2 O and activated carbon, mixed with appropriate amount of water, dried at 400°C N 2 Roasting in the atmosphere for 5 hours; then according to the Pd loading 0.2% (activated carbon mass fraction), measure H 2 PdCl 4 The solution was mixed with the above-mentioned calcined product, impregnated overnight, and...
Embodiment 3
[0043] Catalyst A preparation. First, weigh Al according to the molar ratio of Cr to Al of 7:3 2 o 3 ·H 2 O and Cr(NO 3 ) 3 9H 2 O, mix evenly with an appropriate amount of water, add a certain amount of ammonium carbonate until the precipitation is complete, then separate and wash until the filtrate is neutral, and then dry the precipitate at 120°C. Shape the dried precipitate at 500°C, N 2 Calcined in the atmosphere for 10 hours to obtain a catalyst precursor; the catalyst precursor was treated with HF at 350° C. for 5 hours to obtain catalyst A.
[0044] Catalyst B, according to AlF 3 Loading capacity 1% (activated carbon mass fraction), weigh Al(NO 3 ) 3 9H 2 O and activated carbon, mixed with appropriate amount of water, dried at 400°C N 2 Roasting in the atmosphere for 5 hours; then according to the Pd loading 0.5% (activated carbon mass fraction), measure the H 2 PdCl 4 The solution was mixed with the above-mentioned calcined product, impregnated overnight,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com