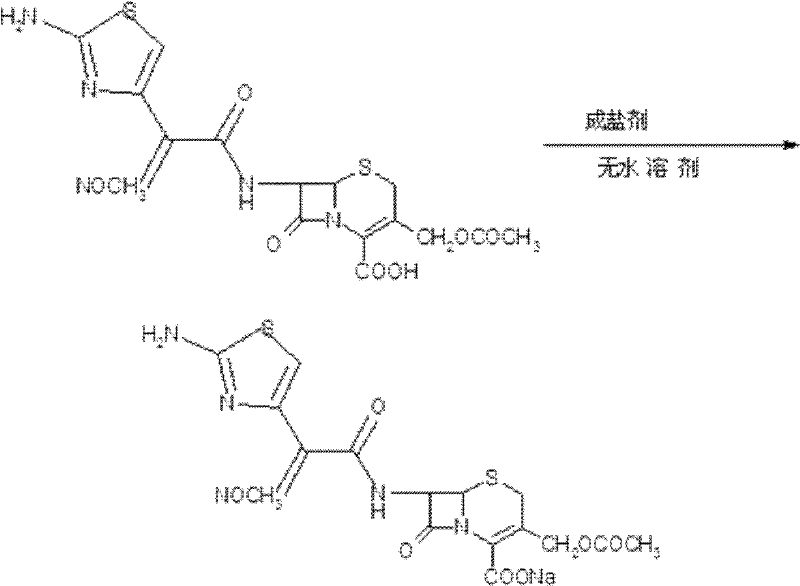
Preparation technology of anhydrous crystal of cefotaxime sodium
A technology of cefotaxime sodium and anhydrous crystallization, which is applied in the field of preparation of pharmaceutical compounds, can solve problems such as high moisture content, easy to exceed the moisture standard, and poor stability, and achieve good color, low moisture content and good stability of the product Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The preparation technology of described cefotaxime sodium anhydrous crystal comprises the steps:
[0020] (1) Add 90ml of anhydrous methanol and 5.95g of sodium methoxide into the three-necked flask, and dissolve in a water bath at 27.5±2.5°C;
[0021] (2) Add 45.5 g of cefotaxime acid, keep warm at 27.5±2.5°C for 2 hours, and the solution becomes clear;
[0022] (3) Add 1 g of activated carbon and stir for 30 minutes to decolorize;
[0023] (4) Fine filtration, top washing with 20ml of anhydrous methanol;
[0024] (5) In a water bath at 27.5±2.5°C, add 900ml of ethyl acetate dropwise to the filtrate to crystallize;
[0025] (6) Suction filtration after crystal growth for 2 hours, washing with ethyl acetate;
[0026] (7) Control the temperature at 60° C. and vacuum-dry at a vacuum degree of 0.095 MPa to obtain 42.93 g of a solid with a yield of 90% and a moisture content of 0.93%.
Embodiment 2
[0028] The preparation technology of described cefotaxime sodium anhydrous crystal comprises the steps:
[0029] (1) Add 90ml of formamide and 20g of sodium isooctanoate into a three-neck flask, and dissolve in an ice-salt bath at -27.5±2.5°C;
[0030] (2) Add 45.5 g of cefotaxime acid, keep warm at -27.5±2.5°C for 2 hours, and the solution becomes clear;
[0031] (3) Add 1 g of activated carbon and stir for 30 minutes to decolorize;
[0032] (4) Fine filtration, top washing with 20ml of cold formamide;
[0033] (5) Under the condition of ice-salt bath -27.5±2.5°C, add 900ml of acetone dropwise to the filtrate to crystallize;
[0034] (6) Suction filtration after crystal growth for 2 hours, wash material with acetone;
[0035] (7) Vacuum drying at 50° C. and vacuum degree of 0.099 MPa to obtain 42.83 g of solid with a yield of 89.7% and a moisture content of 0.90%.
Embodiment 3
[0037] The preparation technology of described cefotaxime sodium anhydrous crystal comprises the steps:
[0038] (1) Add 90ml of dimethyl sulfoxide and 6g of sodium acetate into a three-necked flask, and dissolve in a water bath at 17.5±2.5°C;
[0039] (2) Add 45.5 g of cefotaxime acid, keep warm at 17.5±2.5°C for 2 hours, and the solution becomes clear;
[0040] (3) Add 1 g of activated carbon and stir for 30 minutes to decolorize;
[0041] (4) Fine filtration, top washing with 20ml dimethyl sulfoxide;
[0042] (5) In a water bath at 17.5±2.5°C, add 900ml of ethyl acetate dropwise to the filtrate to crystallize;
[0043] (6) Suction filtration after crystal growth for 2 hours, washing with ethyl acetate;
[0044] (7) Vacuum drying at 60° C. under temperature control and vacuum degree of 0.097 MPa to obtain 42.83 g of solid, yield of 89.7%, and moisture of 0.72%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com