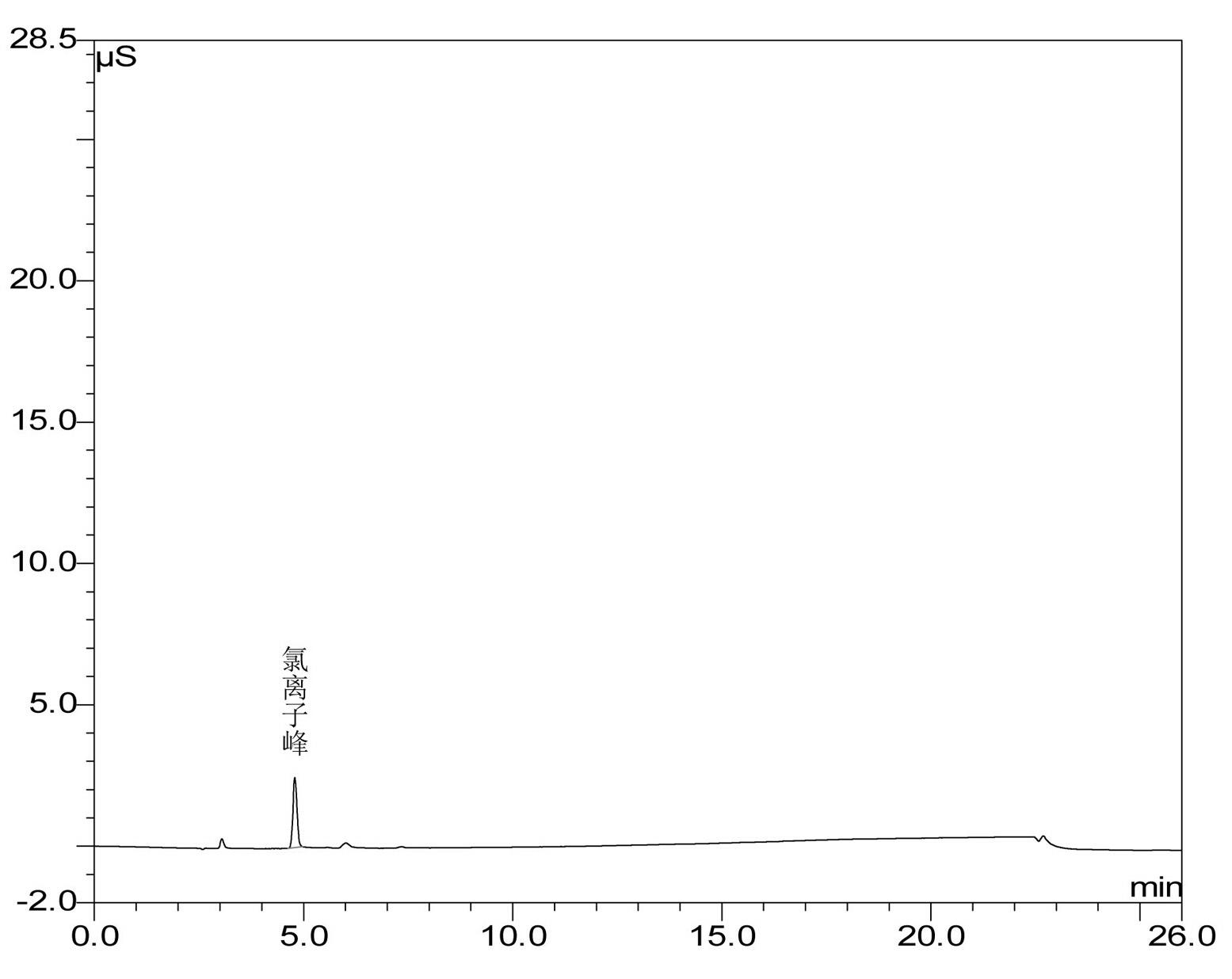
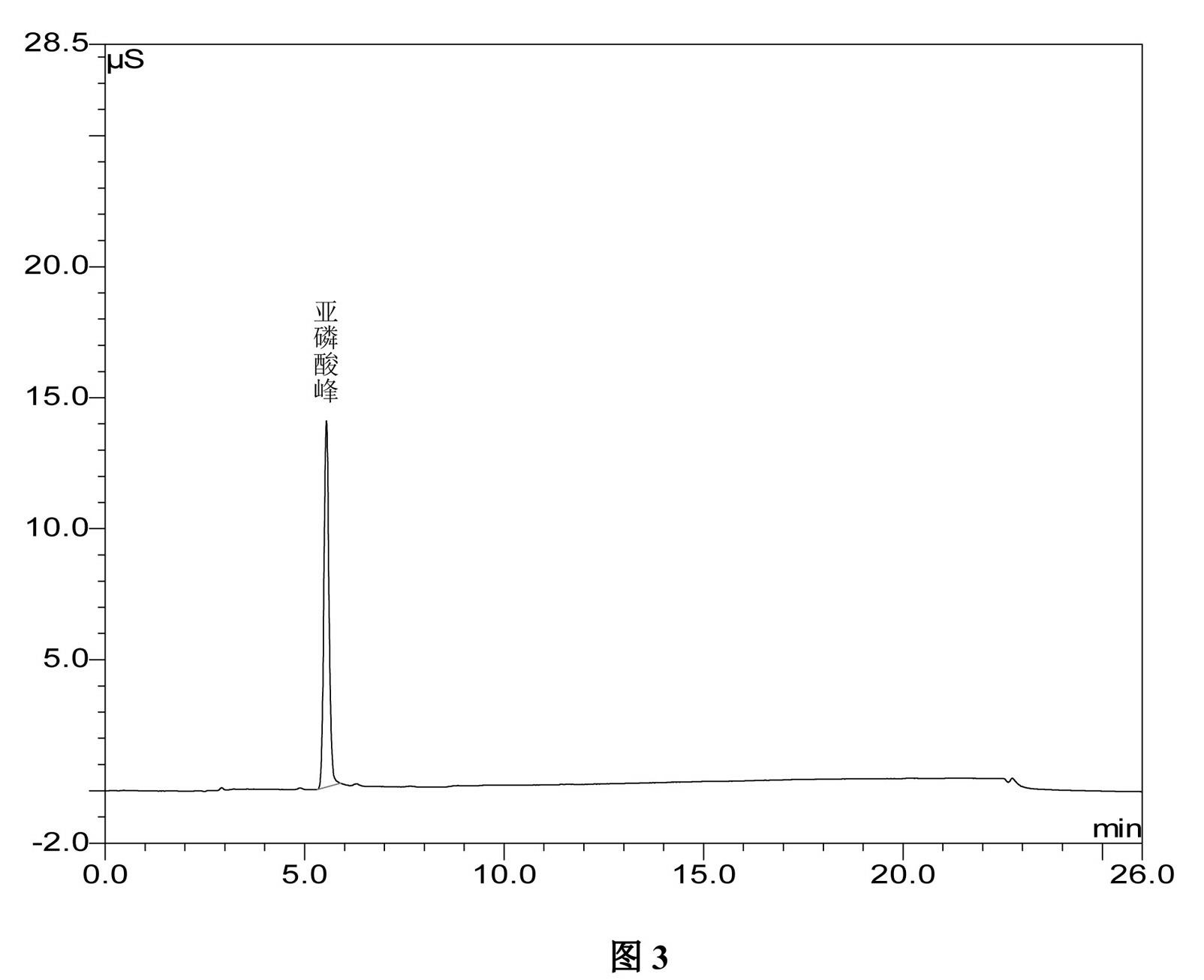
Method for preparing clodronate disodium
A technology of disodium clodronate and clodronate, applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., can solve the problems of low synthesis efficiency, high production cost and long processing time and other problems, to achieve mild reaction conditions, reduce raw material costs, and improve production efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The preparation method of disodium clodronate 1
[0044] 1) Preparation of clodronic acid: a. Weigh 150g of triethyl phosphonite and 100g of dibromomethane, heat and reflux at 180°C for 6 hours, distill under reduced pressure, cut off the fraction at 140~150°C, -0.096 Mpa to obtain colorless Oily liquid I; b. Add 12 times the volume of 10wt% hypochlorous acid solution to 100 g of colorless oily liquid I, then add 3 g of phase transfer catalyst tetrabutylammonium bromide, react at 0 ° C for 1.5 hours, add 2 times the volume of di Extract with methylene chloride, take the dichloromethane extract layer, distill off the dichloromethane under normal pressure to obtain slightly yellow oily liquid II; c. add 4 times the mass of 36.5% hydrochloric acid to the slightly yellowish oily liquid II, stir and reflux for 2 hours, The hydrochloric acid solution was distilled off under reduced pressure to obtain clodronic acid;
[0045] 2) Add appropriate amount of water to dissolve clo...
Embodiment 2
[0047] The preparation method of disodium clodronate 2
[0048] 1) Preparation of clodronic acid: a. Weigh 150g of triethyl phosphonite and 100g of dibromomethane, heat and reflux at 180°C for 6 hours, distill under reduced pressure, cut off the fraction at 140~150°C, -0.096 Mpa to obtain colorless Oily liquid I; b. Add 12 times the volume of 10wt% hypochlorous acid solution to 100 g of colorless oily liquid I, then add 3 g of phase transfer catalyst tetrabutylammonium bromide, react at 0 ° C for 1.5 hours, add 2 times the volume of di Extract with methylene chloride, take the dichloromethane extract layer, distill off the dichloromethane under normal pressure to obtain slightly yellow oily liquid II; c. add 4 times the mass of 36.5% hydrochloric acid to the slightly yellowish oily liquid II, stir and reflux for 2 hours, The hydrochloric acid solution was distilled off under reduced pressure to obtain clodronic acid;
[0049] 2) Add an appropriate amount of water to dissolve ...
Embodiment 3
[0052] The preparation method of disodium clodronate 3
[0053] 1) Preparation of clodronic acid: a. Weigh 100g of triethyl phosphonite and 100g of dibromomethane, heat and reflux at 190°C for 4 hours, distill under reduced pressure, intercept 140~150°C, -0.096 Mpa fraction to obtain colorless Oily liquid I; b. Add 10 times the volume of 10wt% hypochlorous acid solution to 100g of colorless oily liquid I, then add phase transfer catalyst tetrabutylammonium bromide 0.5g + tetrabutylammonium bisulfate 1.0g, 5°C React for 1.5 hours, add 3 times the volume of dichloromethane for extraction, take the dichloromethane extract layer, and evaporate the dichloromethane under normal pressure to obtain a slightly yellow oily liquid II; c. Add 3 times the mass of dichloromethane to the slightly yellowish oily liquid II 20% hydrochloric acid, stirred and refluxed for 3 hours, evaporated the hydrochloric acid solution under reduced pressure to obtain clodronic acid;
[0054] 2) Add an appro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com