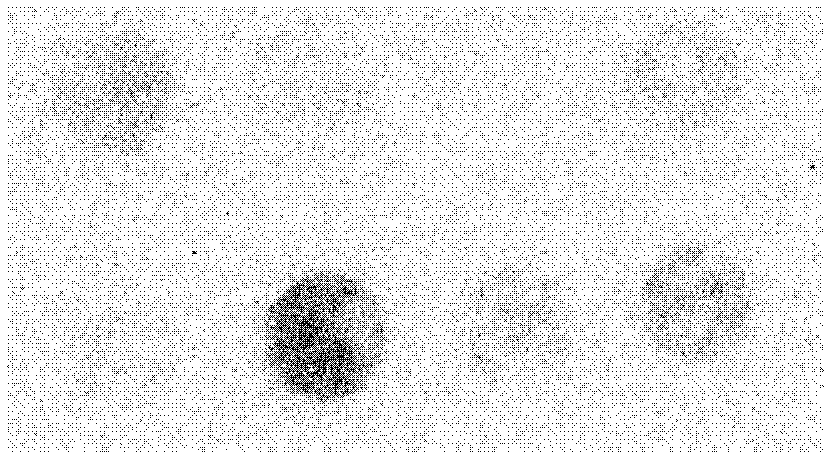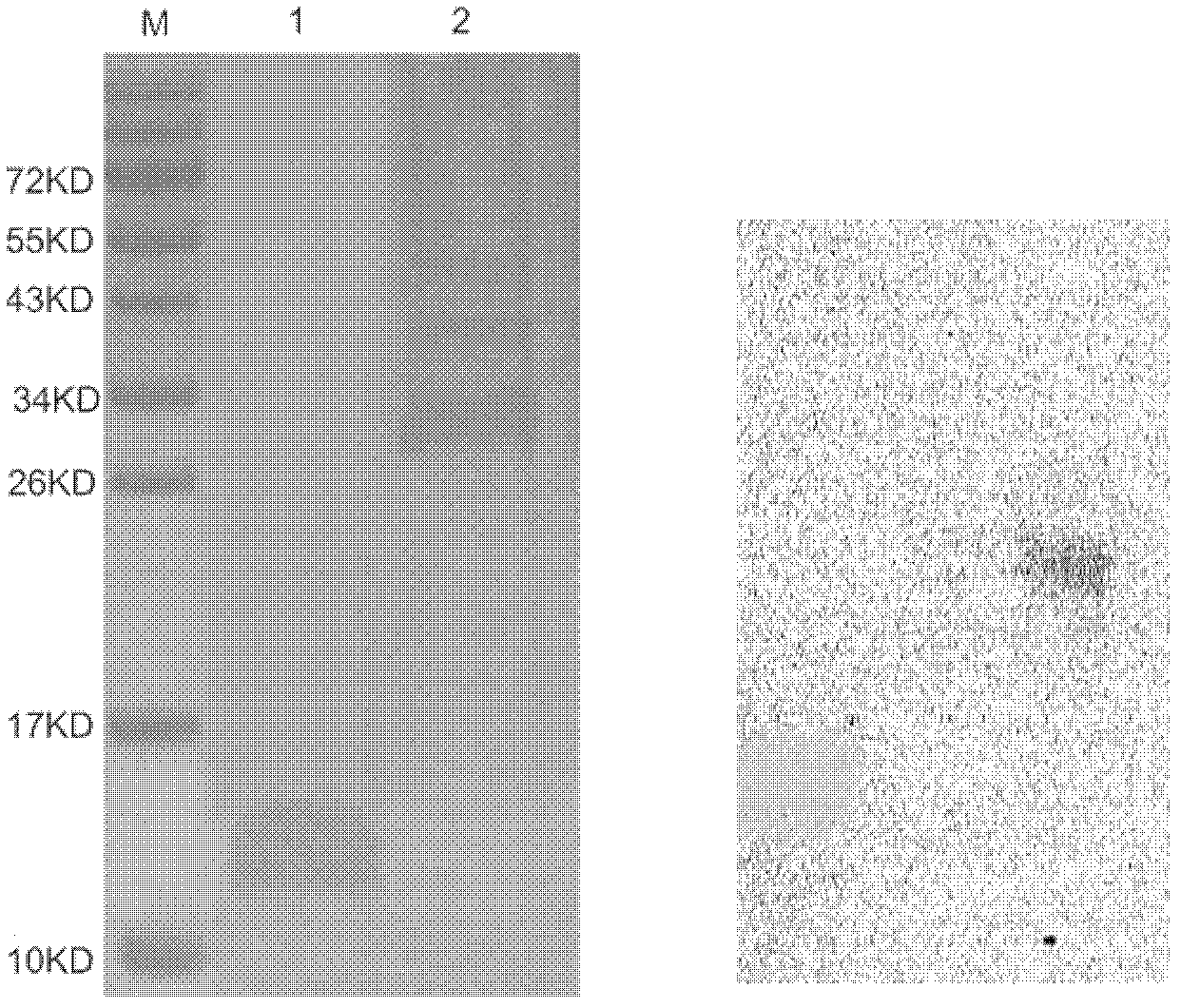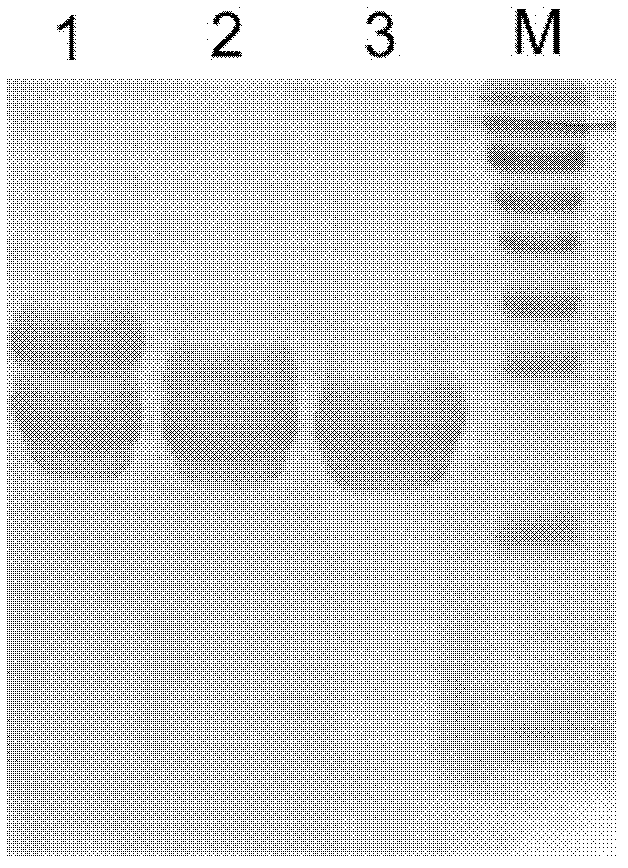Human serum amyloid A1 and preparation method and application thereof
A technology of amyloid protein and human serum, applied in the field of bioengineering, can solve problems such as difficult to obtain SAA1 and difficult to separate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0098] The preparation method of the recombinant human serum amyloid A1 and its multimer of the present invention comprises the steps of:
[0099] (a) providing a yeast expression vector containing a gene encoding human serum amyloid A1;
[0100] (b) providing a yeast host cell containing said yeast expression vector;
[0101] (c) cultivating the yeast host cell under suitable expression conditions, thereby expressing the recombinant human serum amyloid A1 of the present invention, or the multimer of the present invention; and
[0102] (d) isolating the recombinant human serum amyloid A1 or its multimers from the culture product.
[0103] In another preferred example, the gene encoding human serum amyloid A1 is amplified, double-digested and then ligated to a commercially available vector (such as pPIC9K) to obtain the yeast expression vector described in step (a). pPIC9K is a secretory vector for high-efficiency expression of foreign proteins, with a signal peptide α-factor...
Embodiment 1
[0153] Preparation of recombinant hSAA1
[0154] Synthesize the following primers:
[0155] Upstream primer (SEQ ID NO: 3):
[0156] 5'GTCG CTC GAG AAA AGA GAG GCT CGAAGCTTCTTTTTCGTTC 3';
[0157] Downstream primer (SEQ ID NO: 4):
[0158] 5'GTCG GAA TTC TCA GTG GTG GTG GTG GTG GTG GTATTTCTCAGGCAG 3';
[0159]The human mRNA was extracted by conventional methods, and the cDNA obtained after reverse transcription was used as a template. The full-length human hSAA1 gene was amplified by conventional PCR using the above primers. The PCR amplified product was digested with XhoI / EcoRI, and inserted into the downstream of the α-factor signal peptide of the vector pPIC9k (purchased from Invitrogen) that had undergone the same digestion, to obtain the vector pPIC9k-hSAA1. When hSAA1 is secreted and expressed, the α-factor signal peptide will be cleaved, thereby releasing the N-terminus of hSAA1.
[0160] Using the electroporation method, the above-mentioned recombinant expression...
Embodiment 2
[0164] Preparation of hSAA1 polypeptide fragment
[0165] The his6-sumo-tev sequence was gene-synthesized by conventional methods, as shown in SEQ ID NO: 5, and the restriction site XhoI was used to insert the α-factor signal peptide downstream of the vector pPIC9k (Invitrogen Company) to obtain the his6-sumo-tev sequence with his6-sumo- The pPIC9k vector pPIC9k-his6-sumo-tev for tev.
[0166] Using the primers named P27-72 in Table 1, the human hSAA1 gene fragment encoding the polypeptide fragment P27-72 was respectively amplified by conventional PCR method. For the PCR amplification product, after double digestion with XhoI / EcoRI, insert it into the downstream of his6-sumo-tev in the vector pPIC9k-his6-sumo-tev that has undergone the same digestion, and obtain the vector pPIC9k-his6-sumo-tev-hSAA1P27 -72.
[0167] Using the electroporation method, the above-mentioned recombinant expression vector pPIC9k-his6-sumo-tev-hSAA1P27-72 was transferred into conventional Pichia pas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com