Novel water-soluble sulfydryl fluorescent probe, and preparation method and application thereof
A fluorescent probe, water-soluble technology, applied in the field of new water-soluble sulfhydryl fluorescent probes and their preparation, can solve the problems of light instability, low signal-to-noise ratio, easy loss of fluorophores in products, etc., and achieve high sulfhydryl determination sensitivity , good biocompatibility, rapid fluorescence change effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
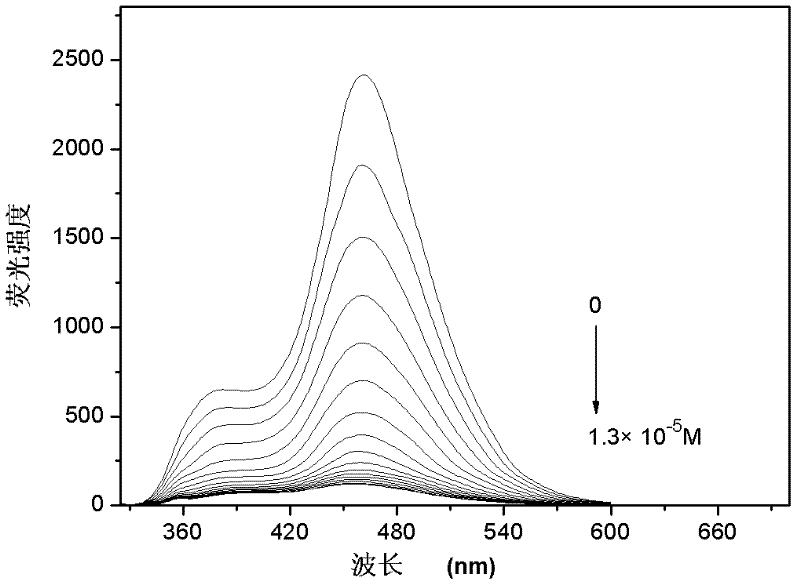
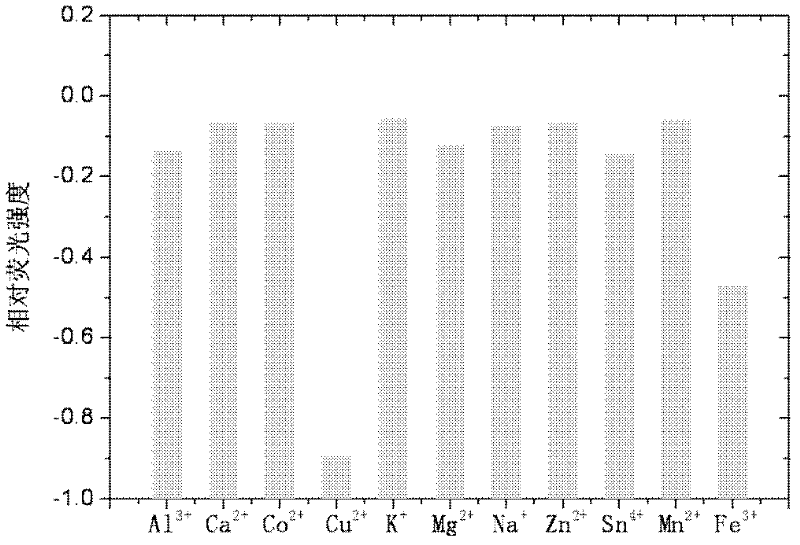
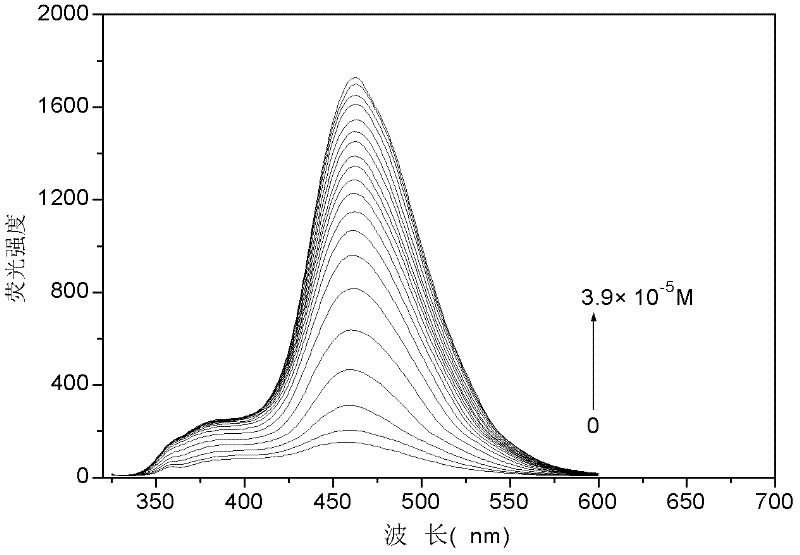
Image
Examples
Embodiment
[0035] A preparation method of the novel water-soluble thiol fluorescent probe, the steps are as follows:
[0036] 1) Dissolve 4.66g of aniline in 40mL of water to obtain aniline aqueous solution, add 15mL of concentrated hydrochloric acid with a mass percentage concentration of 37% to completely dissolve the aniline, cool to 0°C in an ice bath, and obtain aniline hydrochloride solution, and dissolve 3.6g of sodium nitrite Dissolve it in 12mL of water to obtain an aqueous solution of sodium nitrite, cool it to 0°C in an ice bath, and add it dropwise to the above-mentioned aniline hydrochloride solution at a rate of 2 drops per second to prepare aniline diazonium salt solution;
[0037] 2) adding 300mL of 1% NaOH aqueous solution into 6.1g salicylaldehyde to obtain a salicylaldehyde solution, and adding the aniline diazonium salt solution dropwise to the salicylaldehyde alkali aqueous solution at a rate of 3 drops per second at 0°C In, dropwise add the Na that the mass percenta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com