Novel mercapto fluorescence probe and uses thereof
A fluorescent probe and sulfhydryl technology, applied in the field of fluorescent probes, can solve the problems of unstable light, difficult fluorescence imaging, low signal-to-noise ratio, etc., and achieve the effects of high sulfhydryl determination sensitivity, rapid fluorescence change, and enhanced fluorescence intensity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: the synthetic route of probe 1 and probe 2:
[0043](1) Preparation of arylamine diazonium salt: Weigh 3-6g of aniline and add it to 40mL of water, then add 15mL of concentrated hydrochloric acid to completely dissolve the aniline into hydrochloride, and cool to 0°C in an ice bath. Weigh 2.3-5g of sodium nitrite and dissolve it in a small amount of water to form a solution, cool with ice, slowly add to the above solution while stirring, and then the benzenediazonium chloride solution can be prepared. Use potassium iodide test paper to test whether it turns blue. If it turns blue, it means that the solution contains excess nitrous acid, which can be neutralized with urea until the potassium iodide test paper does not change color;
[0044] (2) Preparation of 5-azophenyl salicylaldehyde: Weigh 2-4g NaOH and dissolve it in 300mL water, add it into a three-necked bottle containing 5-10g salicylaldehyde, and dissolve it into salicylaldehyde sodium salt in wate...
Embodiment 2
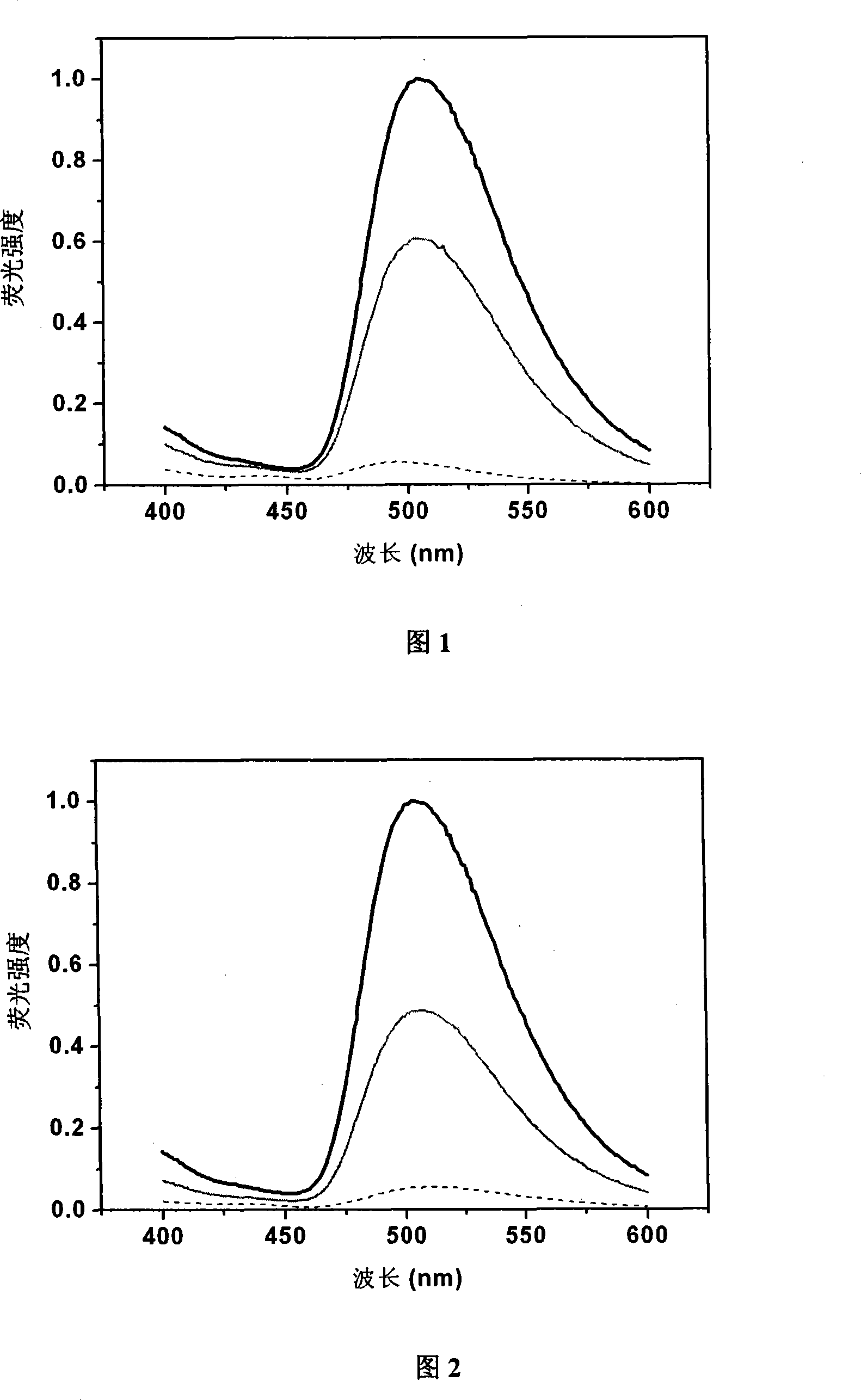
[0057] Embodiment 2: Fluorescence spectrum change of probe 1 and probe 2 reacting with thiol compound:
[0058] Dissolve probe 1 and probe 2 in DMSO to a final concentration of 100 μM, take 200 μl and react with 1 mM thiol-containing compound L-cysteine (L-Cys) for 1, 5 min, then measure the change in fluorescence intensity, The wavelength is 336nm. The results can be seen in Figure 1 and Figure 2, and all fluorescence intensities were normalized.
[0059] It can be seen from Figure 1 and Figure 2 that the background fluorescence intensity of these two probes is very low, and after reacting with the thiol compound L-Cys, the fluorescence intensity is significantly enhanced within 1 minute (probe 1: 11.4 times, probe 2: 8.9 times), the maximum emission wavelength of fluorescence is at 505nm. After five minutes of reaction, the fluorescence tended to be stable, the fluorescence intensity of probe 1 was enhanced by 19.5 times, and the fluorescence intensity of probe 2 was enh...
Embodiment 3
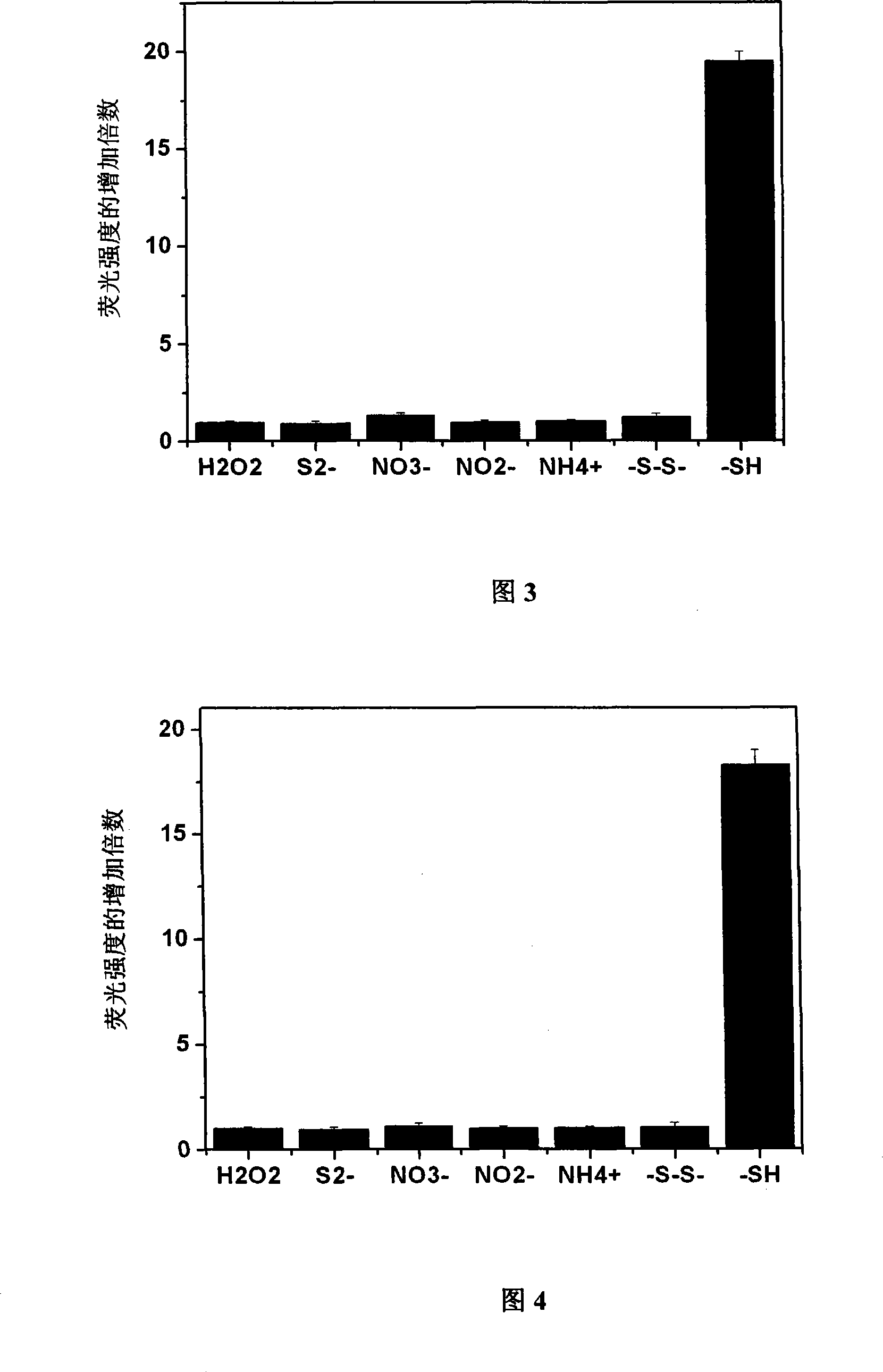
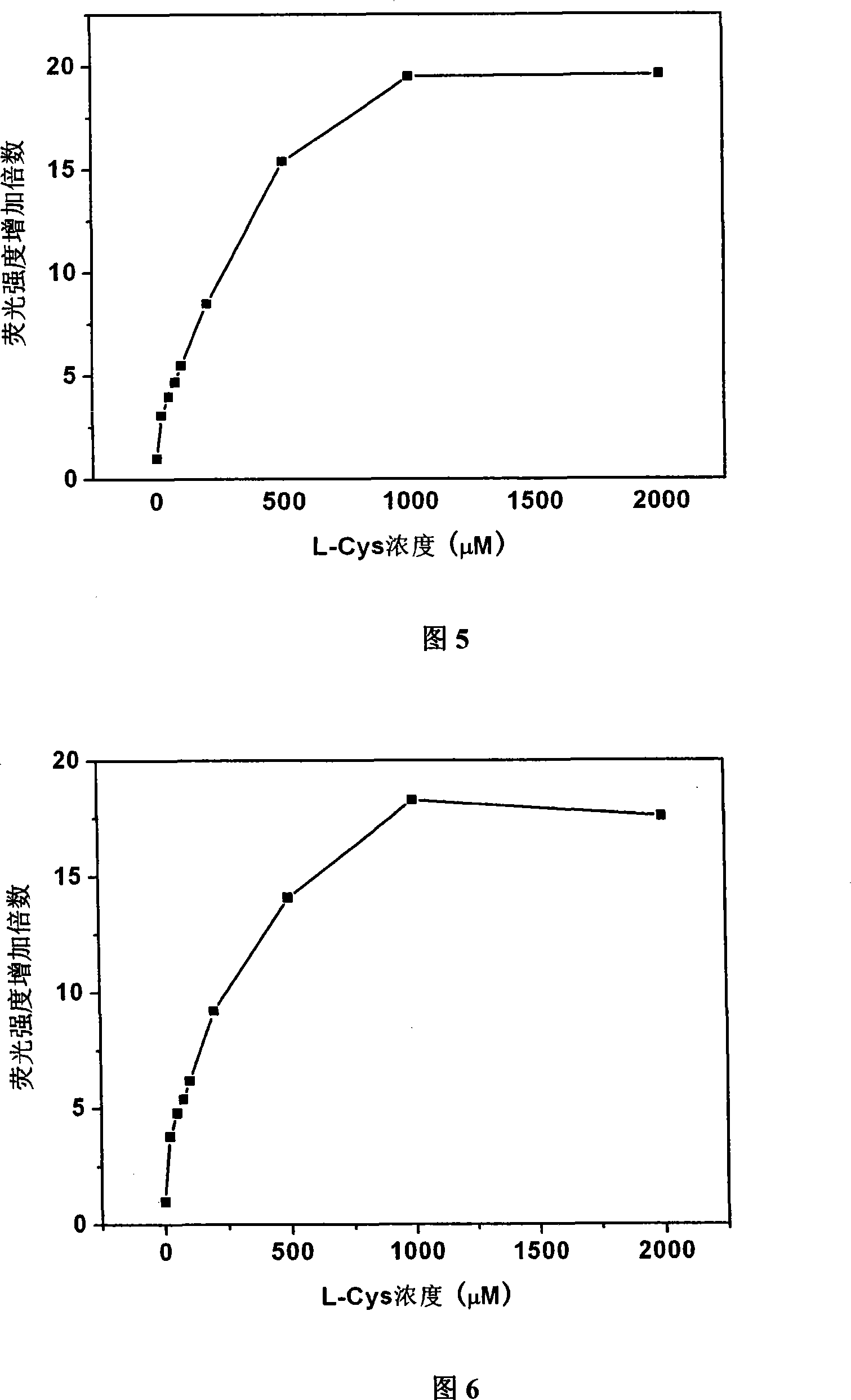
[0060] Embodiment 3: the selectivity of probe to thiol
[0061] Probe 1 and probe 2 were taken and dissolved in DMSO to a concentration of 1 mM. Add two kinds of probes to the 96-well plate, add hydrogen peroxide, sodium sulfide, nitrate, nitrite, ammonium salt, cystine, and L-Cys respectively, and then add deionized water to all samples, The final concentration of probes obtained in each well was 100 μM, and the molar amounts of hydrogen peroxide, sodium sulfide, nitrate, nitrite, ammonium salt, cystine, and L-Cys were 100 times that of the corresponding probes, and the total The volume is 150 μl. After reacting for 1 hour, measure the fluorescence intensity at 505nm on a microplate reader, and divide the fluorescence intensity after the reaction by the fluorescence intensity of the original probe to obtain the fluorescence enhancement multiple after the reaction, and then determine the pairing of probe 1 and probe 2. Specificity of sulfhydryl reaction. The results are sho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com