Method for removing CO2 and H2S out of synthesis gas by utilizing amine-type solid absorbent
An absorbent and synthesis gas technology, applied in separation methods, chemical instruments and methods, gas fuels, etc., can solve the problems of high energy consumption and corrosion of synthesis gas, achieve stable and reliable operation, avoid heat loss, investment and operating costs cheap effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
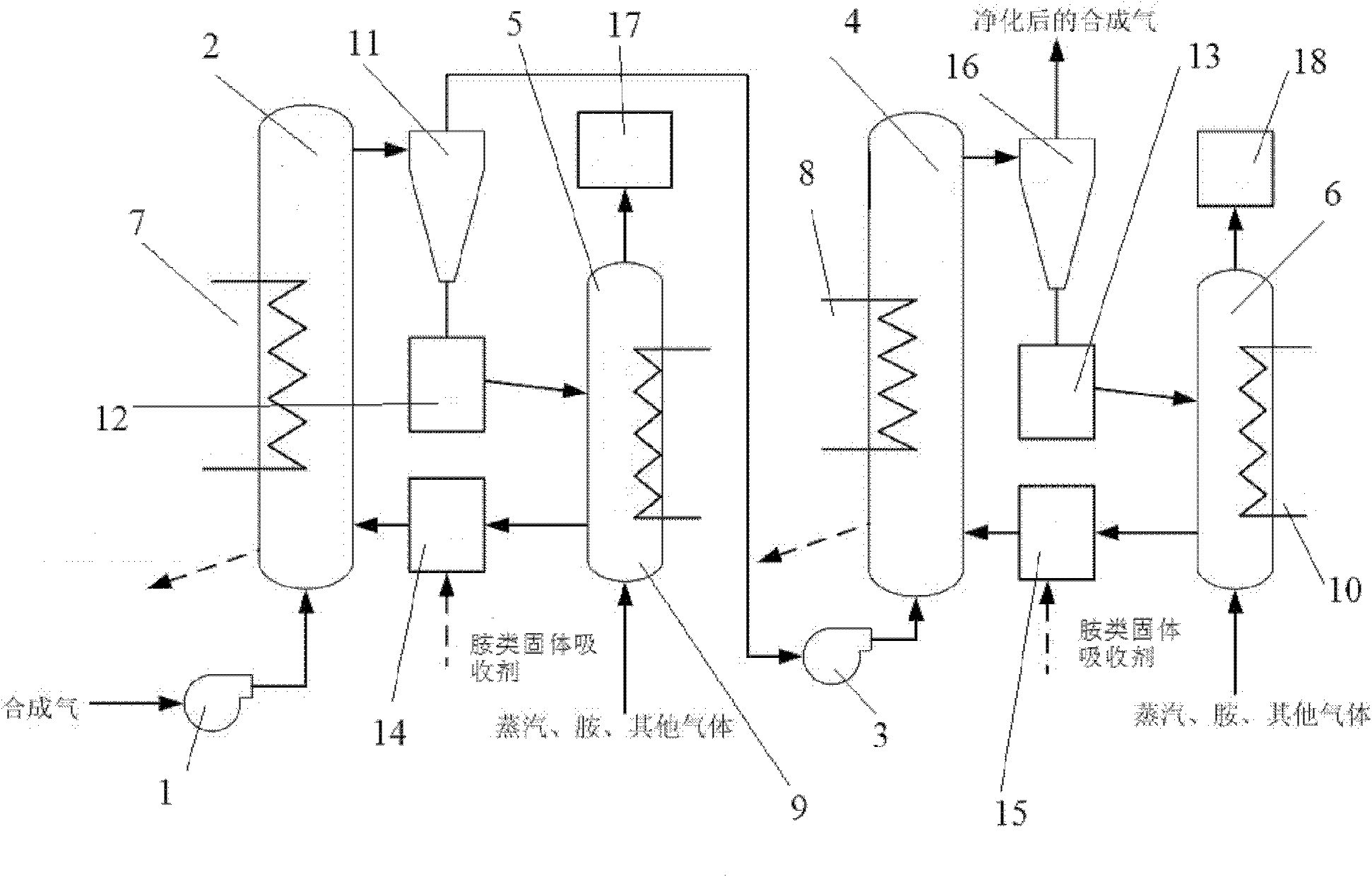
[0039] A method to remove CO from syngas by using amine solid absorbent 2 and H 2 The method of S is carried out according to the following steps:
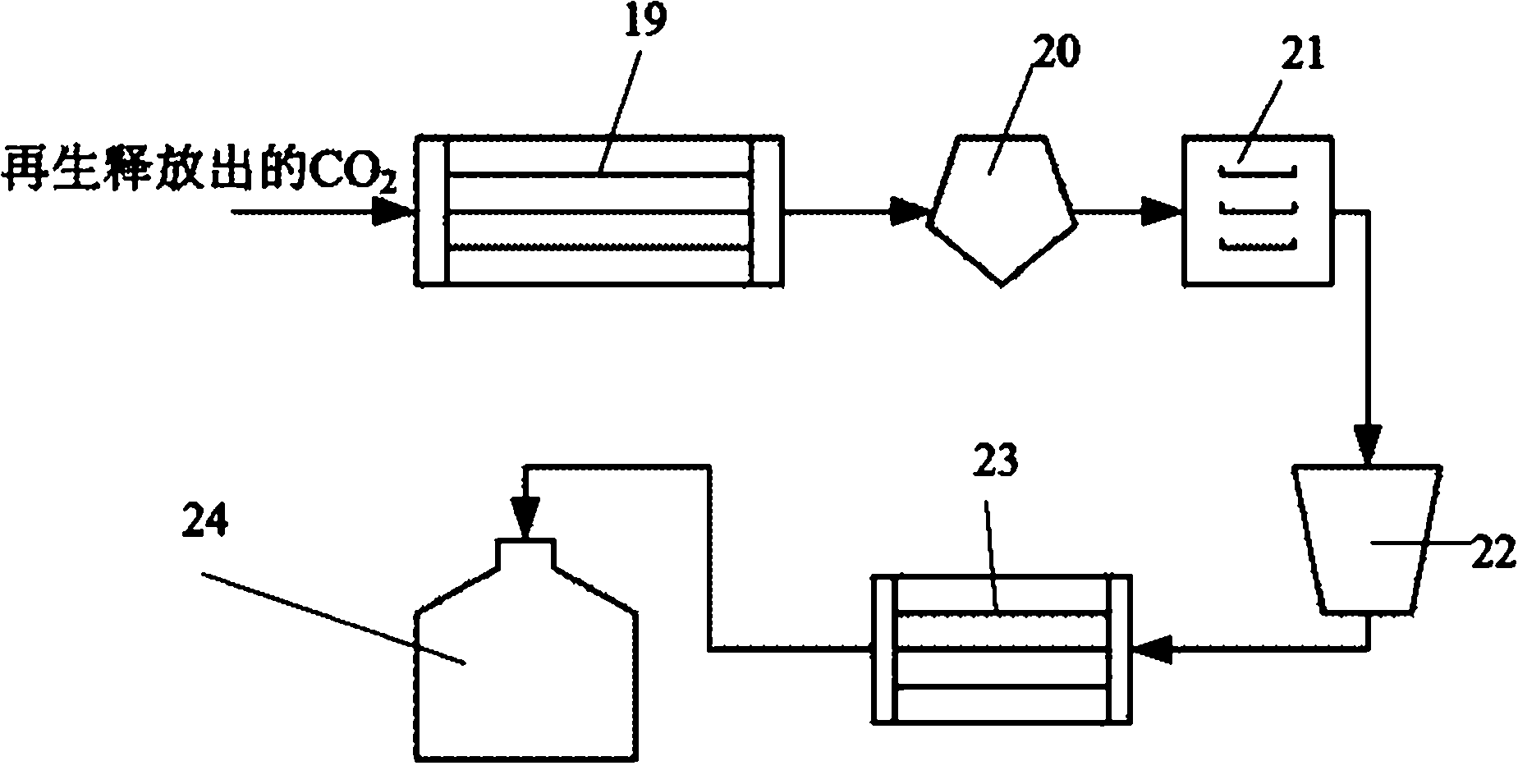
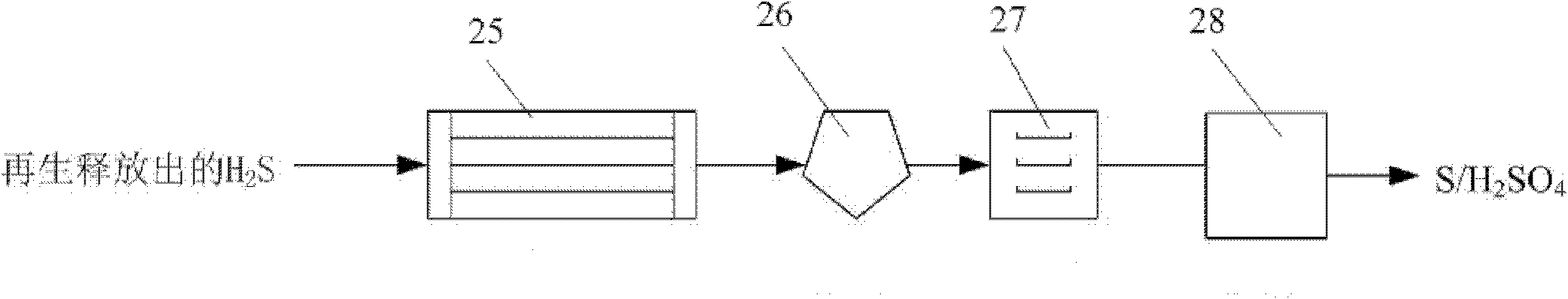
[0040] First, after dedusting, the syngas enters the CO2 containing amine solid absorbent through the first blower 1. 2 Absorption reactor 2, adjust its temperature to 50-60°C, the CO in the syngas 2 Absorbed by amine solid absorbents, the decarburized syngas is separated by the first gas-solid separator 11 and enters the second stage H through the second blower 3 2 S absorbs reactor 4, adjusts its temperature to be 15-20 ℃, and the separated solid matter enters the first solid return device 12, wherein the H 2 S is absorbed by amine solid absorbents. The desulfurized synthesis gas enters the second gas-solid separator 16 for separation, and the separated H 2 S is for subsequent use. absorbed CO 2 、H 2 The amine solid absorbent of S enters the first regeneration reactor 5 and the second regeneration reactor 6 respectively ...
Embodiment 2
[0047] First, through the dust removal, the synthetic gas enters the CO containing amine solid absorbent through the third blower 29. 2 and H 2 S Absorption Reactor 30, CO in Syngas 2 and H 2 S is absorbed by the amine solid absorbent, and the desulfurized and decarbonized synthesis gas is separated by the third gas-solid separator 32 and then enters the third regeneration reactor 34 through the third solid return valve 33, and the regeneration method is to feed CO 2 or H 2 S or water vapor is heated at the same time to increase the temperature of the regeneration reactor, so that the CO absorbed by the amine solid absorbent 2 and H 2 S can be effectively desorbed, so that the amine solid absorbent can be reused, and the regenerated amine solid absorbent returns to the CO through the third gas seal and solid return device 36. 2 and H 2 S absorption reactor 30; the purified syngas from the third gas-solid separator 32 enters subsequent utilization, and the CO from the thi...
Embodiment 3
[0052] First, through the dust removal, the synthetic gas enters the first layer of absorption reaction zone 41 of the absorption reactor containing amine solid absorbent through the fourth blower 40, and the CO in the synthetic gas 2 Absorbed by the amine solid absorbent, the decarburized synthesis gas enters the second layer of absorption reaction zone 43 through the second layer, and the H in the synthesis gas 2 S is absorbed by amine solid absorbents. The syngas after desulfurization and decarburization and the solid absorbent in the first and second layers enter the fourth gas-solid separator 45, the separated syngas enters the subsequent utilization, and the solid absorbent enters the fourth solid absorbent through the fourth solid returner 46. Regeneration reactor 48 performs regeneration treatment. The regeneration method is the introduction of CO 2 or H 2 S or water vapor is heated at the same time to increase the temperature of the regeneration reactor, so that th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com