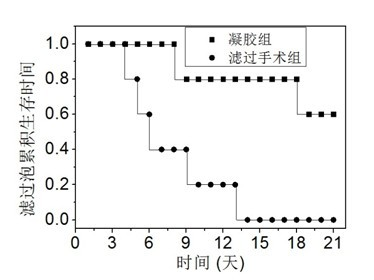
Preparation method of glycopeptide hydrogel containing glucosamine unit and application of glycopeptide hydrogel to preparing postoperation scar inhibitor
A glucosamine and hydrogel technology, applied in the field of biomedicine, can solve the problems of increased cost and difficulty in artificial synthesis of decorin, and achieve the effect of saving cost and simplifying the treatment process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
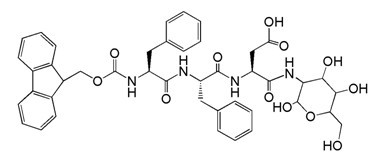
[0033] (1) Preparation of tripeptide FMOC-Phe-Phe-Asp(OtBu)-OH containing hydrophobic N-fluorene-9-methoxycarbonyl
[0034] Using 2-chloro-trityl chloride resin (substitution degree of available chlorine on the resin is 1.32mmol / g) as a solid phase carrier, the short peptide FMOC-Phe-Phe-Asp (OtBu) was prepared by using a peptide solid-phase automatic synthesizer -OH. The peptide chain extends from the carbon-terminus to the nitrogen-terminus on the resin. The specific synthesis steps are as follows: Weigh 1.5g of 2-chloro-trityl chloride resin (the available chlorine content is 1.5×1.32=1.98mmol), and use 10mL of dichloromethane (CH 2 Cl 2 ) and N,N-dimethylformamide (DMF) for 3 washes, and then soak the resin with 10 mL of DMF for 30 minutes. Remove the DMF, add the DMF solution dissolved in FMOC-Asp(OtBu)-OH (3×1.98mmol) and diisopropylethylamine (DiEA, 4×1.98mmol) to the resin, shake and react at room temperature for 30 minutes . The reaction solution was removed, the...
Embodiment 2
[0049] (1) Preparation of tripeptide Nap-Phe-Phe-Asp(OtBu)-OH containing hydrophobic naphthyl acetyl group
[0050] With reference to the method of Example 1, the peptide chain was extended on the 2-chloro-trityl chloride resin. After the synthesis of the peptide chain was completed, a solution of naphthaleneacetic acid (2×1.98mmol), DiEA (3×1.98mmol), HBTU ( 2.4×1.98mmol) and HOBt (2.4×1.98mmol) in DMF were added to the resin, and the reaction was shaken at room temperature for 1.5 hours. Aspirate the DMF, respectively with 10mL DMF and CH 2 Cl 2 Wash the resin 3 times and dry the resin at room temperature. Add 20 mL of cutting agent (1:2:7 volume ratio of acetic acid, trifluoroethanol and CH 2 Cl 2 ) to cut the product from the resin and collect the filtrate and concentrate. The product was precipitated by adding a large amount of cold diethyl ether, filtered and washed several times, and dried overnight at room temperature.
[0051] (2) Synthesis of glycopeptide Nap-P...
Embodiment 3
[0057] (1) Preparation of tripeptide Py-Phe-Phe-Asp(OtBu)-OH containing hydrophobic pyrenebutyryl
[0058] Referring to the method of Example 1, extend the peptide chain on the 2-chloro-trityl chloride resin. After the synthesis of the peptide chain is completed, add pyrenebutyric acid (2×1.98mmol), DiEA (3×1.98mmol), HBTU (2.4×1.98mmol) and HOBt (2.4×1.98mmol) in DMF were added to the resin, and the reaction was shaken at room temperature for 1.5 hours. Aspirate the DMF, respectively with 10mL DMF and CH 2 Cl 2 Wash the resin 3 times and dry the resin at room temperature. Add 20 mL of cutting agent (1:2:7 volume ratio of acetic acid, trifluoroethanol and CH 2 Cl 2 ) to cut the product from the resin and collect the filtrate and concentrate. The product was precipitated by adding a large amount of cold diethyl ether, filtered and washed several times, and dried overnight at room temperature.
[0059] (2) Synthesis of glycopeptide Py-Phe-Phe-Asp-glucosamine containing glu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com