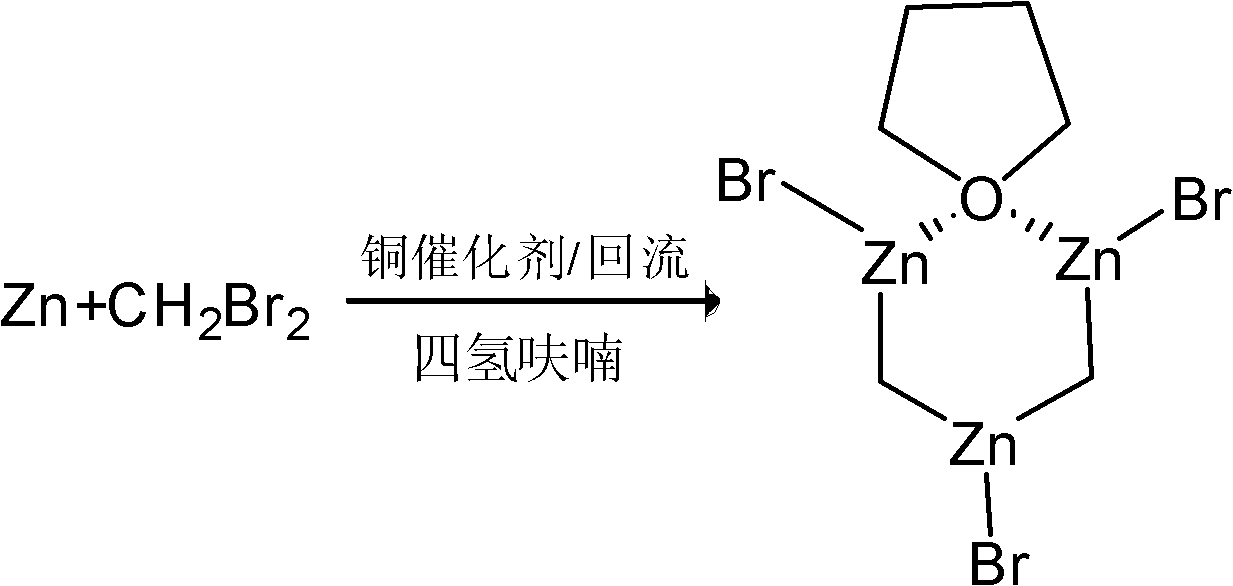
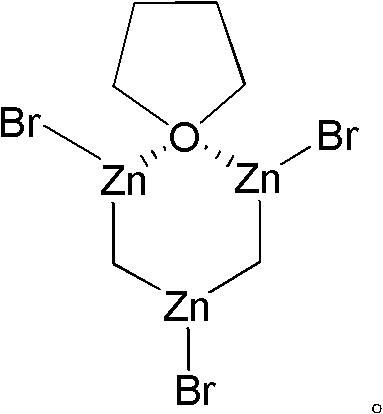
Preparation method of nysted reagent
A technology of Nast reagent and solvent, which is applied in the field of fine chemicals, can solve the problems of long synthesis cycle, high price, large amount of dichloroethane, etc., and achieve the effect of simple and practical method, less environmental pollution and good economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] A preparation method of Nastel reagent, the steps are as follows:
[0024] 1) Add 4.9g of zinc powder to 5.4g of methanol solvent under the protection of nitrogen, and add 5.72g of isopropanol solution with a mass percentage concentration of 12% hydrogen chloride under stirring. The rate of dripping rate is 3 to 5 drops per second. The temperature of the addition was controlled at 20-25°C. After the addition was completed, the resulting mixture was heated at reflux temperature for 2h, cooled to room temperature, and allowed to stand for 20 minutes, then the supernatant solution was poured out, and the remaining solid was heated to 50- 60℃, evaporate to dryness under reduced pressure by a water pump to obtain activated zinc powder;
[0025] 2) Add the above activated zinc powder and 0.025g copper catalyst to 19g tetrahydrofuran, then slowly add 6.9g dibromomethane dropwise for the first time, the mixture is heated at reflux temperature for 16h, then cooled to room temperature...
Embodiment 2
[0027] A preparation method of Nastel reagent, the steps are as follows:
[0028] 1) Add 4.9g of zinc powder to 5.4g of methanol solvent under the protection of nitrogen, and add dropwise 2.84g of isopropanol solution with a concentration of 19% hydrogen chloride by mass percentage under stirring. The drip rate is 3 to 5 drops per second. The temperature of the addition was controlled at 20-25°C. After the addition was completed, the resulting mixture was heated at reflux temperature for 2h, cooled to room temperature, and allowed to stand for 20 minutes, then the supernatant solution was poured out, and the remaining solid was heated to 50- 60℃, evaporate to dryness under reduced pressure by a water pump to obtain activated zinc powder;
[0029] 2) Add the activated zinc powder and 0.025g of copper catalyst to 19g of tetrahydrofuran, then slowly add 6.9g of dibromomethane dropwise for the first time, the mixture is heated at reflux temperature for 16h, then cooled to room temperat...
Embodiment 3
[0031] A preparation method of Nastel reagent, the steps are as follows:
[0032] 1) Add 4.9 zinc powder to 18.7g methanol solvent under the protection of nitrogen, add 5.72g of isopropanol solution with a mass percentage concentration of 12% hydrogen chloride under stirring conditions, and the dropping rate is 3 to 5 drops per second. The temperature is controlled at 20-25℃. After the addition is completed, the resulting mixture is heated at reflux temperature for 2h, cooled to room temperature, and allowed to stand for 20 minutes, then the supernatant solution is poured out, and the remaining solid is heated to 50-60 ℃, evaporate to dryness under reduced pressure by a water pump to obtain activated zinc powder;
[0033] 2) Add the activated zinc powder and 0.025g of copper catalyst to 19g of tetrahydrofuran, then slowly add 6.9g of dibromomethane dropwise for the first time, the mixture is heated at reflux temperature for 16h, then cooled to room temperature, and then slowly drop...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com