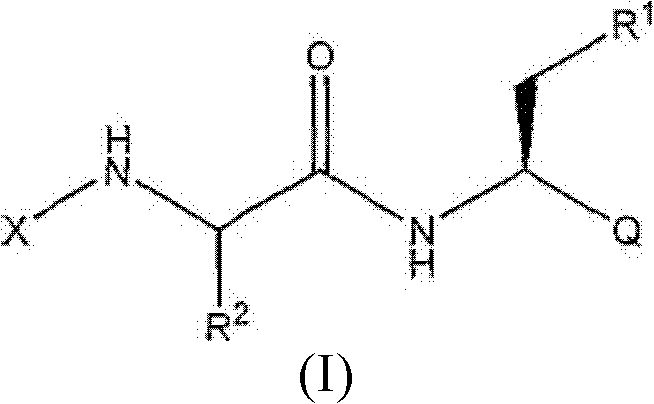
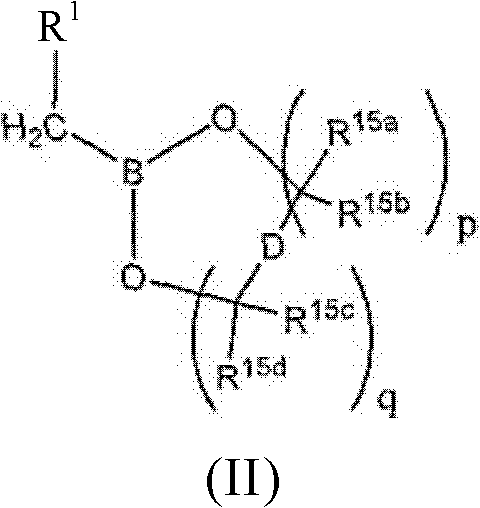
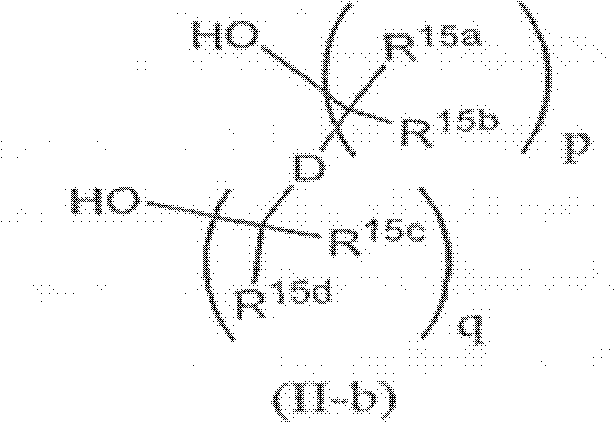Proteasome Inhibitors and Methods of Using the Same
A technology of medicinal salts and alkyl groups, applied in the field of boronic acid and boronic ester compounds, can solve problems such as reducing the degradation rate of animal p53 protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A1
[0503] (1R)-1-[(3aS,4S,6S,7aR)-hexahydro-3a,5,5-trimethyl-4,6-methylene-1,3,2-benzodi Synthesis of borolan-2-yl]-3-methylbutylamine hydrochloride
[0504] Step 1: 2-(2-Methylpropyl)-(3aS,4S,6S,7aR)-hexahydro-3a,5,5-trimethyl-4,6-methylene-1,3,2 -Benzodi Borolane e
[0505]
[0506] A mixture of (+)-pinanediol (23.9g, 0.140mol) and 2-methylpropylboronic acid (15g, 0.147mol) in diethyl ether (300ml) was stirred at room temperature for 24 hours. The mixture was dried over anhydrous sodium sulfate and purified by column chromatography (silica gel 230-400 mesh), eluting with a 90:10 mixture of hexane:ethyl acetate. A clear oily product was obtained (32.6 g, yield 94%).
[0507] 1 H NMR(DMSO-d 6 ): 4.28 (1H, dd, J = 8.8 Hz, 2.0); 2.30 (1H, m); 2.18 (1H, m); 1.96 (1H, t, J = 5.3); 1.86 (1H, m); 1.78 ( 1H, set, J = 6.8); 1.68 (1H, m); 1.30 (3H, s); 1.25 (3H, s); 1.01 (1H, d); 0.9 (6H, d, J = 6.6); 0.81 ( 3H, s); 0.69 (2H, m).
[0508] Step 2: 2-[(1S)-1-chloro-3-methylbutyl]-(3aS,4S,6S,7...
Embodiment A2
[0521] 2-(2-Methylpropyl)-(3aS,4S,6S,7aR)-hexahydro-3a,5,5-trimethyl4,6-methylene-1,3,2-benzodioxa Alternate synthesis of borocyclopentane e
[0522] Step 1: 2-(1-Methylethoxy)-(3aS,4S,6S,7aR)-hexahydro-3a,5,5-trimethyl-4,6-methylene-1,3, 2-benzodi Borolane e.
[0523]
[0524] To a solution of (1S, 2S, 3R, 5S)-(+)-pinanediol (50.0g, 0.293mol) in anhydrous tetrahydrofuran (350ml) was slowly added triisopropoxyborane while maintaining at 0°C Stir under nitrogen. After 2 hours, the solvent was removed by rotary evaporation. The oily residue was redissolved in hexane (150 ml), and the solution was filtered to remove a small amount of white solid. The filtrate was concentrated by rotary evaporation to obtain a clear oily product (62.6 g, yield 90%).
[0525] 1 H NMR (DMSO-d6): 4.31-4.20 (2H, m); 2.34-2.16 (2H, m); 1.96 (1H, t, J=5.5); 1.90-1.85 (1H, m); 1.74-1.67 ( 1H, m); 1.32 (3H, s); 1.31 (1H, d, J = 7.6); 1.25 (3H, s); 1.14 (3H, d, J = 6.1); 1.13 (3H, d, J = 6.1 ); 0.81 (3H, ...
Embodiment B1
[0530] N-[(1S)-1-[[[(1R)-1-[(3aS,4S,6S,7aR)-hexahydro-3a,5,5-trimethyl-4,6-methylene- 1,3,2-benzodi Borolan-2-yl]-3-methylbutyl]amino]carbonyl]-4-[[imino(nitroamino)methyl]amino]butyl-carbamic acid 1,1-dimethyl Ethyl ester
[0531]
[0532] Method A: HOAt / HATU
[0533] To BocNH(NO 2 ) ArgOH (15.7g, 49.3mmol) in anhydrous DMF (100ml) solution was added HATU (O-(7-azabenzotriazol-1-yl)-1,1,3,3-tetramethyluronium Hexafluorophosphate; 18.7 g, 49.3 mmol) and HOAt (1-hydroxy-7-azabenzotriazole; 6.71 g, 49.3 mmol). The mixture was cooled to 0°C and N-methylmorpholine (13.6ml, 0.123mol) was added. After 10 minutes, add (1R)-1-[(3aS,4S,6S,7aR)-hexahydro-3a,5,5trimethyl-4,6-methylene-1 of Example A.1, 3,2-benzodi Borolan-2-yl]-3-methylbutylamine hydrochloride (12.4 g, 41.1 mmol). The cooling bath was removed and the mixture was stirred at room temperature for 4.5 hours. The mixture was diluted with ethyl acetate (800ml), with 2% citric acid solution (2×150ml), 2% NaHCO 3 Wash with so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com