Synthesis method of calcium ion selective chelating agents
A synthetic method and selective technology, applied in the field of synthesis of calcium ion selective chelating agent, can solve the problems of cumbersome steps, low product purity and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
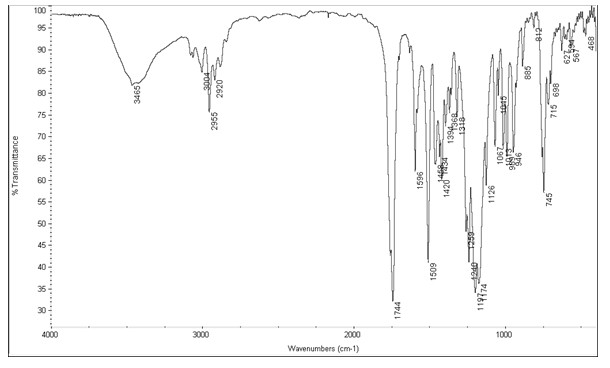
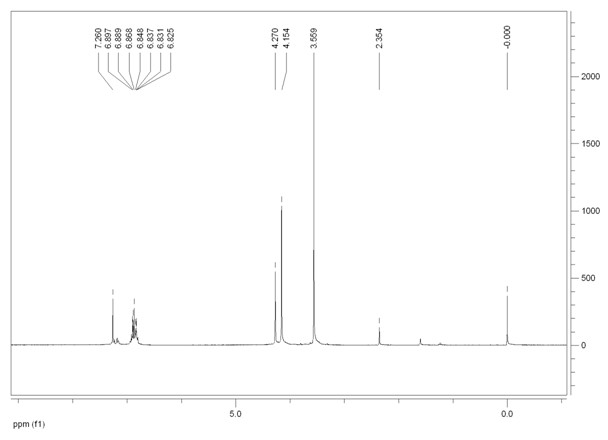
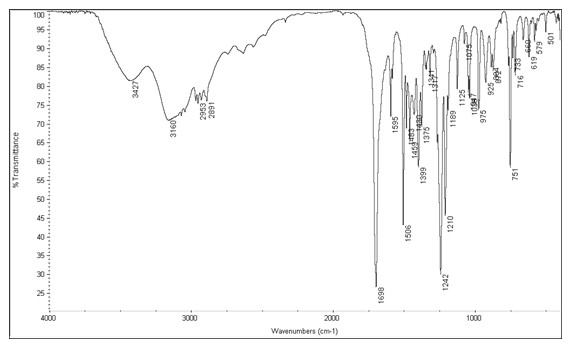
Image
Examples
Embodiment Construction
[0020] The synthetic method of compound 1a comprises the following steps:
[0021] 1. The synthetic reaction steps of compound 1a:
[0022] The first step: add 19.52g (80mmol) 1,2-bis(2-aminophenoxy)ethane, 3.344g (62.4mmol) anhydrous sodium iodide, 72ml (416mmol) of diisopropylethylamine and 120ml of acetonitrile.
[0023] The second step: Slowly heat up and stir. The temperature of the internal bath was raised to 55°C, and 38.4ml (416mmol) of methyl bromoacetate was added dropwise at a constant speed.
[0024] Step 3: after the dropwise addition, the system is filled with nitrogen and sealed, and the temperature is raised to 80° C. in the system again, and then refluxed for 20 hours.
[0025] 2. Post-processing steps for compound 1a synthesis
[0026] Step 4: After the reflux is completed, add 140ml of toluene into the four-neck flask, stir for 30 minutes, and then let stand for 30 minutes. After standing, pour the upper clear layer into a 500ml beaker, add 50ml of tolu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com