High purity cyclic peptide compound and preparation method and application thereof
A compound and high-purity technology, applied in the field of high-purity cyclic peptide compounds and their preparation, can solve problems such as low product purity, ineffective removal of impurities, and large amounts of organic solvents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0293] Preparation of crude compound I
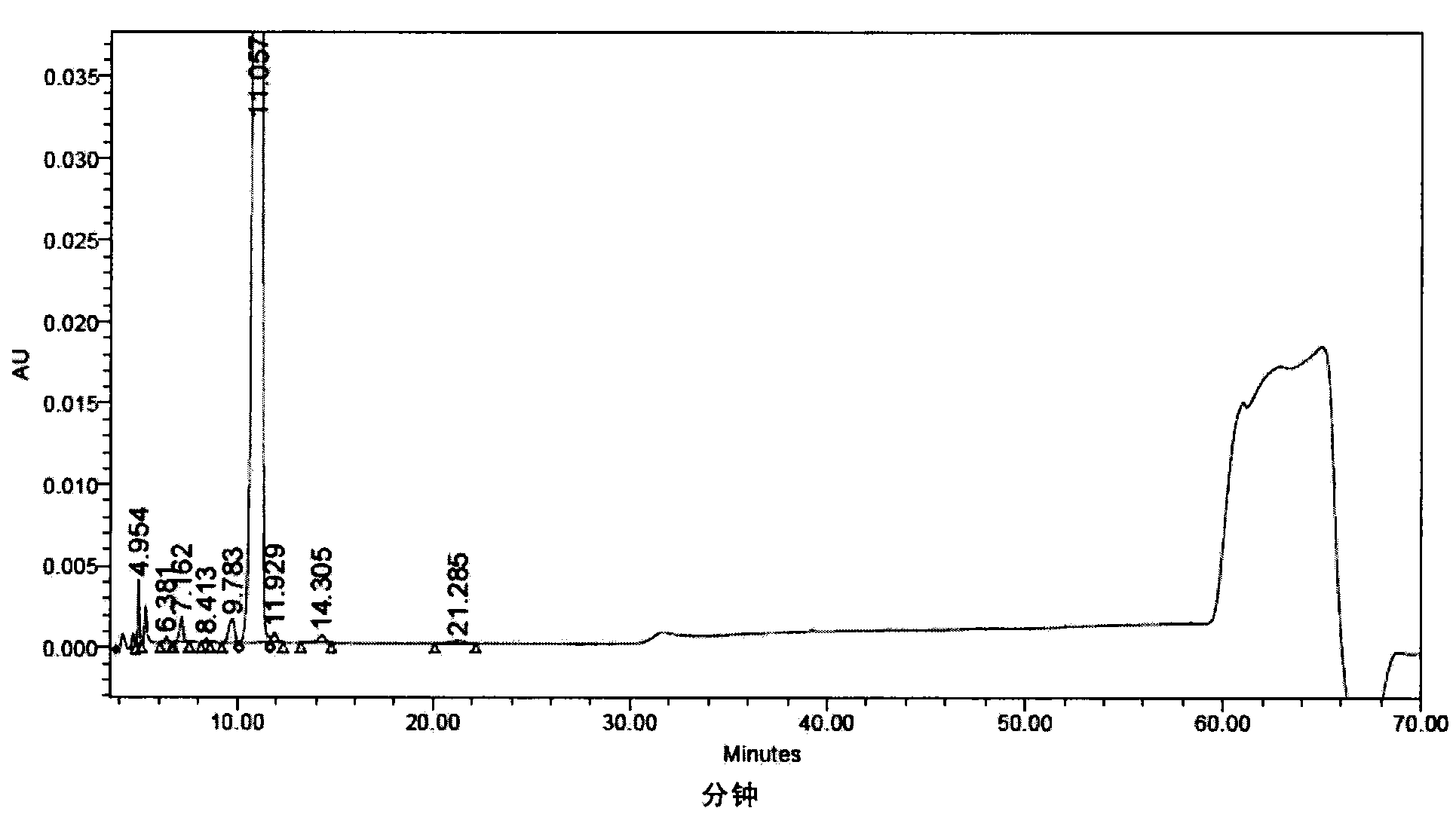
[0294] Referring to the method of Example 1 of US Patent No. 5,376,634, 76 g of solid powder of Compound I was prepared, and the content determined by HPLC was 97.51%, see figure 1 HPLC analysis spectrum.
[0295] Table 1
[0296] Impurity name
Embodiment 2
[0298] Preparation of high-purity formula I compound
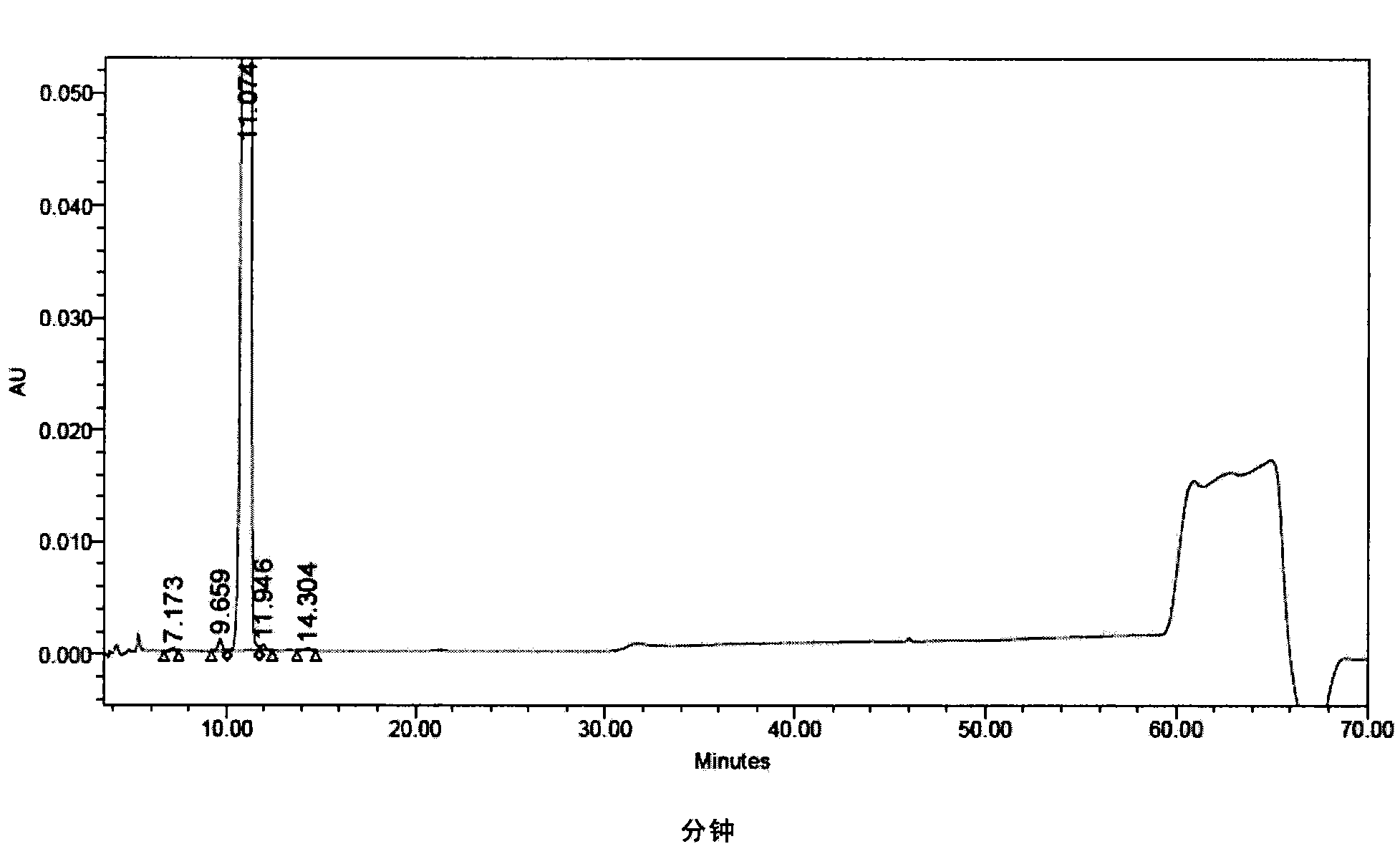
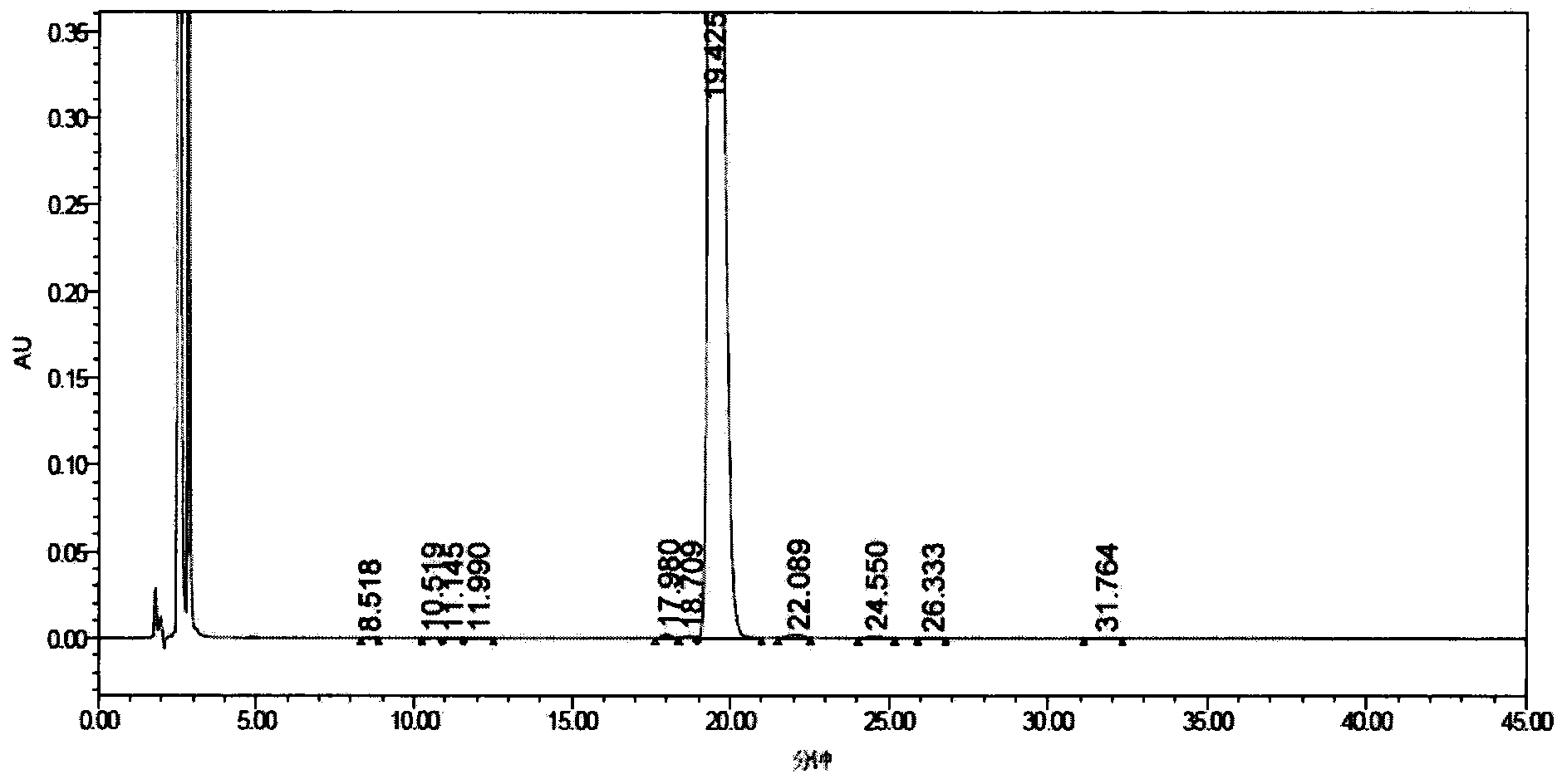
[0299] At 30° C., 3.6 g of the crude compound I prepared in Example 1 was dissolved in a mixed solution of 25 ml of water and 20 ml of n-propanol, and stirred to completely dissolve the compound I. Glacial acetic acid was used to adjust the pH to 3.5, and the solution was cooled to 15°C, the crystals of Compound I were precipitated, and the stirring was continued at 15°C for 5 hours, so that the crystals of Compound I gradually grew. 90 ml of n-propanol was added dropwise, and after completion of the dropwise addition, the mixture was stirred at 15° C. for 1 hour. Suction filtration and vacuum drying afforded 3.5 g of compound I with a purity of 99.00% as determined by HPLC. The contents of the main relevant impurities are shown in Table 2.
[0300] Table 2
[0301] Impurity name
Embodiment 3
[0303] Preparation of high-purity formula I compound
[0304] At 40°C, 3.5g of Compound I prepared in Example 2 (Compound I prepared in Example 2, with an HPLC purity of 99.00%) was dissolved in a mixed solution of 19ml of water and 16ml of n-propanol, and stirred to make the compound I dissolve completely. Glacial acetic acid was used to adjust the pH to 2.0, and the solution was cooled to 15°C, the crystals of compound I were precipitated, and the stirring was continued at 15°C for 5 hours, so that the crystals of compound I grew gradually. 70 ml of n-propanol was added dropwise, and after the dropwise addition was completed, the mixture was stirred at 15° C. for 1 hour. Suction filtration and vacuum drying afforded 3.4 g of compound I with a purity of 99.23% as determined by HPLC. The content of relevant impurities is shown in Table 3.
[0305] table 3
[0306] Impurity name
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com