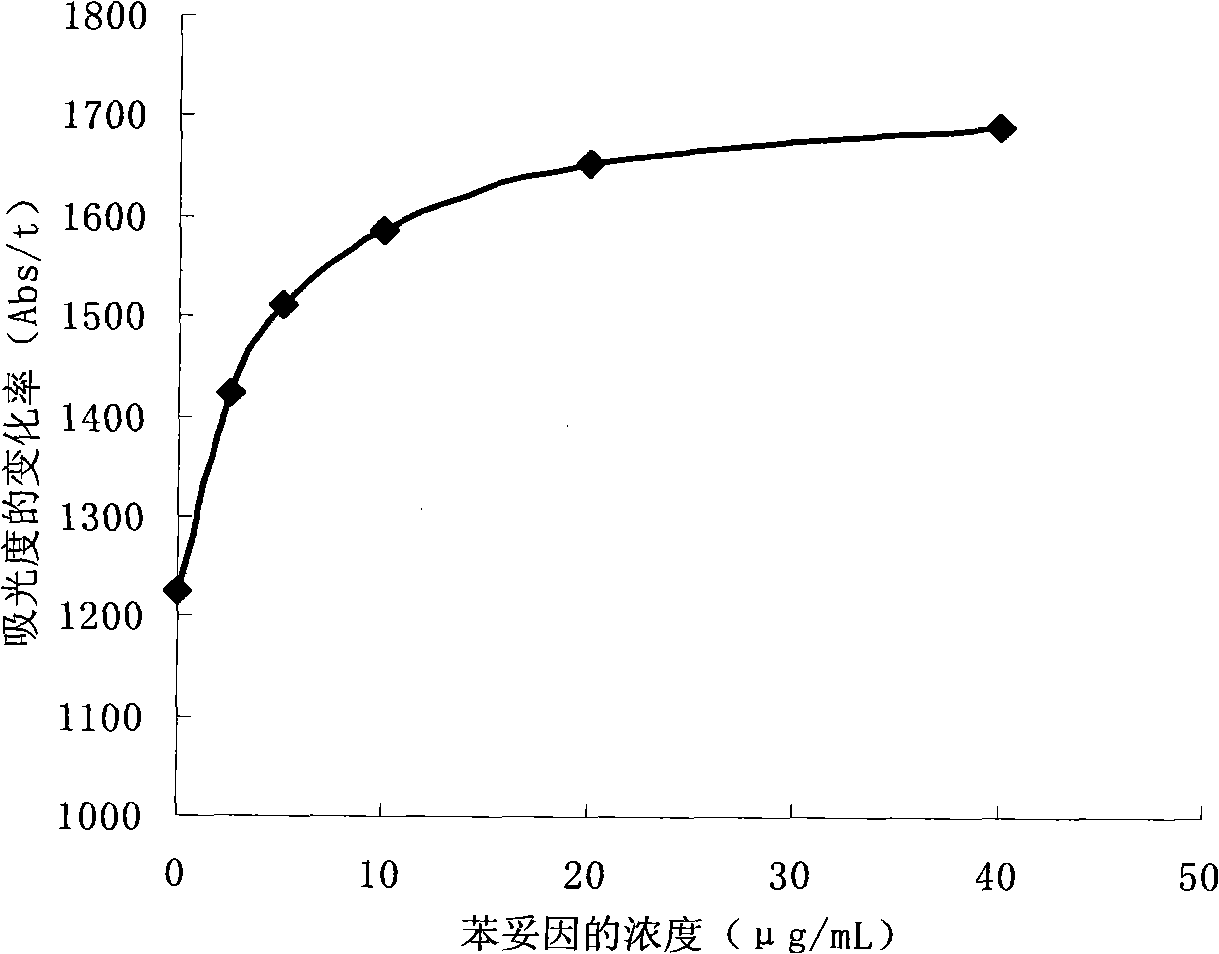
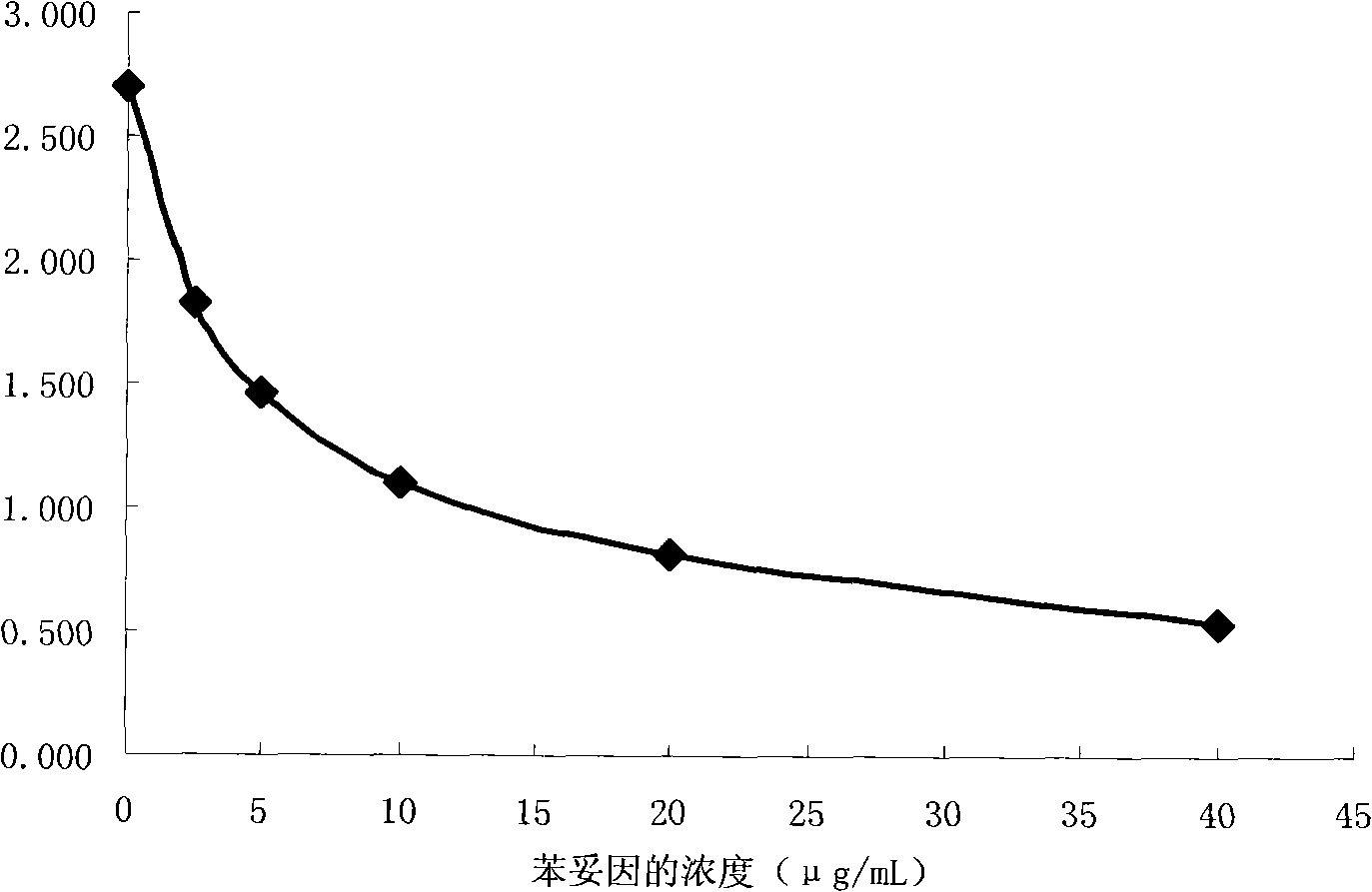
Methods for detecting phenytoin
A detection method and phenytoin technology, applied in the biological field, can solve the problems of not reaching the effective blood drug concentration, narrow therapeutic window, large individual differences, etc., and achieve the effects of convenient operation, strong specificity and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Example 1 Synthesis of phenytoin immunogen
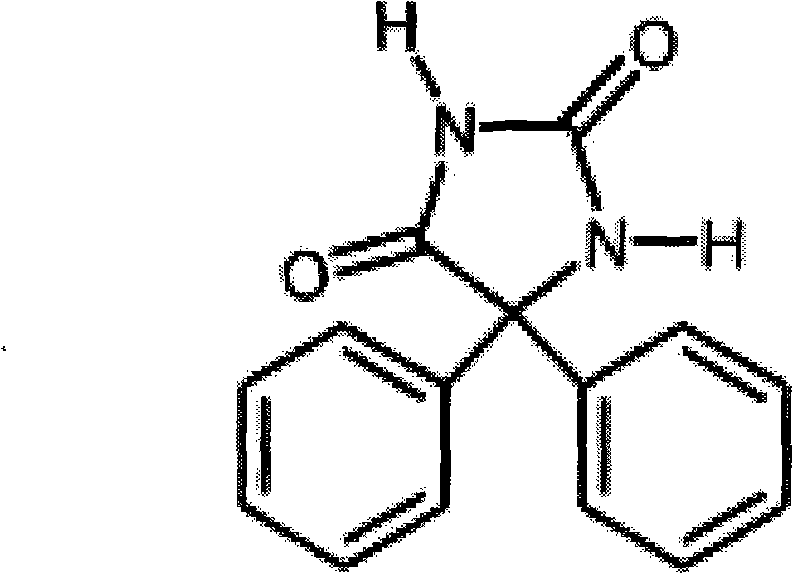
[0078] The chemical structure of the phenytoin derivative used in the following examples is shown in formula (III).
[0079]
[0080] Formula (III)
[0081] 1. the synthesis of phenytoin derivatives, its chemical structural formula is as shown in formula (III)
[0082] (1) Synthetic Chemicals 3
[0083]
[0084] 1) Accurately weigh 5.3g, 26.6mmol of compound 1 (hydroxybenzophenone) and 1.9g, 14mmol of anhydrous potassium carbonate (K 2 CO 3 ), these two compounds were added to the flask.
[0085] 2) Add 150 mL of DMF and sonicate for 15 min to dissolve the compound.
[0086] 3) Add 6.5g, 31.2mmol of chemical compound 2 (ethyl 5-bromopentanoate), and stir at 55°C for 72h.
[0087] 4) Filter the solution after cooling down to room temperature, dissolve the reactants with 5mL DMF, and dry in vacuo.
[0088] 5) Use 150mL concentrated sodium bicarbonate (NaHCO 3 ) solution to dissolve the residue, extract with 150mL ...
Embodiment 2
[0116] Example 2 Preparation of Anti-Phenytoin Specific Antibody
[0117] (1) Dilute the synthetic phenytoin immunogen to 1.5 mg / ml with PBS to obtain an antigen solution, then mix the antigen solution with Freund's complete adjuvant, and inject it into rabbits;
[0118] (2) After 2 to 3 weeks, inject the rabbit once with 1.0ml of the same antigen solution mixed with Freund's incomplete adjuvant, and then once every four weeks, twice in total, and extract the antiserum of the rabbit to obtain effective antibodies.
Embodiment 3
[0119] Example 3 A homogeneous enzyme immunoassay for phenytoin
[0120] 1. Preparation of homogeneous enzyme immunoassay reagents
[0121] (1) Preparation of R1 reagent
[0122] Dilute the prepared antibody into R1 buffer, the homogeneous R1 buffer contains 50mM Tris, 0.25% BSA, 50mM G-6-P and 50mM NAD. The volume ratio of antibody to R1 buffer was 1:2000.
[0123] (2) Preparation of R2 reagent
[0124] 1) Preparation of G6PDH-phenytoin
[0125] a) Weigh 15mg G6PDH, dissolve it in 12ml, 0.05M Tris buffer, add 100mg NADH, 0.5ml carbitol and 1ml DMF in sequence and mix well;
[0126] b) Dissolve 10 mg of phenytoin derivative in 420 μl DMSO and 180 μl DMF, add 6 μl tributylamine and 3 μl isochloroformate, and stir for 30 minutes at 2-8°C;
[0127] c) Stir overnight at 2-8° C., and purify the obtained G6PDH-phenytoin.
[0128] 2) Dilute the prepared G6PDH-phenytoin into R2 buffer. R2 buffer was 100 mM Tris, 0.25% BSA. The volume ratio of antibody to R2 buffer was 1:3000. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com