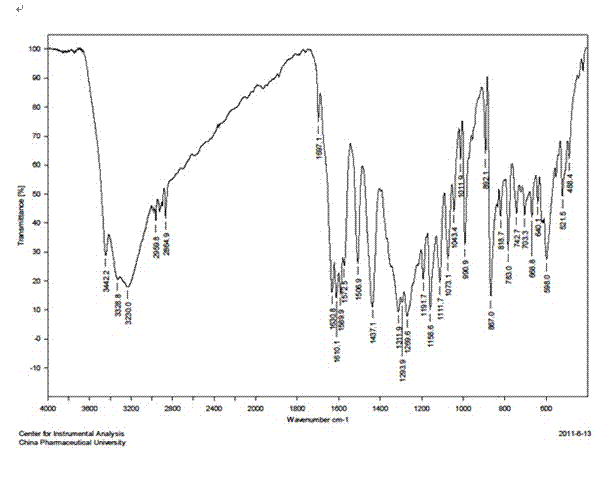
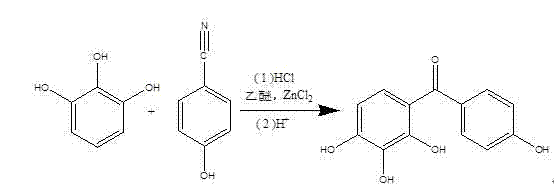
Method for synthesizing 2, 3, 4, 4'-tetrahydroxybenzophenone (THBP)
A technology of tetrahydroxybenzophenone and p-hydroxybenzonitrile, which is applied in the field of synthesizing 2,3,4,4'-tetrahydroxybenzophenone, can solve the problems of complicated post-treatment process, high reaction temperature and poor environment. Friendly and other issues, to achieve the effect of reducing the amount of catalyst used, high catalytic activity, and reducing pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] (1) Put 31.5 g (ie 0.25 mol) of pyrogallic acid, 150 ml of anhydrous ether and 28.6 g (ie 0.24 mol) of p-hydroxybenzonitrile into the reactor, stir at room temperature, and wait until the solid is completely dissolved, Add ZnCl 2 13.6 g (that is, 0.1 mol ), and immediately pass through dry hydrogen chloride gas, stop passing through hydrogen chloride after 3 hours, react at room temperature for 24 hours, and then pass through hydrogen chloride for 2 hours. After that, 48 ml of 20 wt% hydrochloric acid aqueous solution and 100 ml of diethyl ether were added, and heated to reflux for 2 h. Naturally cooled to room temperature after the reaction, the organic layer was separated, the aqueous layer was extracted 3 times with ether (23 ml each time), and the organic layers were combined. The obtained organic layer material was washed 3 times with water, and the anhydrous MgSO 4 Dry, filter with suction, and concentrate under reduced pressure to obtain 60.05 g of crude 2,3,4...
Embodiment 2
[0020] Example 2: Put 31.5 g (0.25 mol) of pyrogallic acid, 150 ml of anhydrous ether and 26.8 g (0.22 mol) of p-hydroxybenzonitrile into the reactor, stir at room temperature, and add ZnCl 2 12.25 g (that is, 0.09 mol ) and immediately pass through dry hydrogen chloride gas, stop passing through hydrogen chloride after 3 hours, react at room temperature for 26 hours, and then pass through hydrogen chloride for 2 hours. After that, 54 ml of 20 wt% hydrochloric acid aqueous solution and 100 ml of diethyl ether were added, and heated to reflux for 2 h. Naturally cooled to room temperature after the reaction, the organic layer was separated, the aqueous layer was extracted 3 times with ether (26ml each time), and the organic layers were combined. The organic layer was washed 3 times with water, and then washed with anhydrous MgSO 4 Dry, filter with suction, and concentrate under reduced pressure to obtain 58.36 g of crude 2,3,4,4'-tetrahydroxybenzophenone.
Embodiment 3
[0022] (1) Put 63.0 g (0.50 mol) of pyrogallic acid, 300 ml of anhydrous ether and 50.4 g (0.42 mol) of p-hydroxybenzonitrile into the reactor, stir at room temperature, and add ZnCl after the solid is completely dissolved. 2 29.8g (that is, 0.22 mol), and immediately pass through dry hydrogen chloride gas, stop passing through hydrogen chloride after 3 hours, react at room temperature for 20 hours, and then pass through hydrogen chloride for 2 hours. After that, 110 ml of 20 wt% hydrochloric acid aqueous solution and 200 ml of diethyl ether were added, and heated to reflux for 2 h. Naturally cooled to room temperature after the reaction, the organic layer was separated, the aqueous layer was extracted 3 times with ether (52 ml each time), and the organic layers were combined. The organic layer was washed three times with water and then washed with anhydrous MgSO 4 After drying, suction filtration and concentration under reduced pressure, 110.63 g of crude 2,3,4,4'-tetrahyd...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com