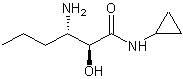
Chiral intermediate (S, S)-3-amino-N-cyclopropyl-2-hydroxyalkanamide or its salt and preparation method thereof
A technology of hydroxycaproylamide and cyclopropyl, applied in the field of preparation of chiral intermediate (S,S)-3-amino-N-cyclopropyl-2-hydroxycaproylamide or its salt, can solve the problem Stability, difficult crystallization and other problems, to achieve high yield, low cost, and reduce energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 1.5 g of water and 30 g of the compound of formula II into a 250 ml three-necked reaction flask, then add 23 g of D-tartaric acid and 90 ml of ethanol, add 40 ml of acetic acid under stirring, heat up and reflux for about 2 hours, then cool down to 25-30°C and stir for crystallization. After filtering, the filter cake was soaked in ethanol, then filtered, and dried to constant weight to obtain 22.3 g of the crude compound of formula III as a white solid, EE: 87.2%, yield: 41%.
[0029]
Embodiment 2
[0031] Add 22.3g of the crude compound of formula III obtained in the example into a mixed solution of 100ml of ethanol and 12ml of water, stir and raise the temperature to 60-65°C, then keep it warm for 45 minutes, then cool down to 20-25°C, crystallize, filter, and the mother liquor Save it for later use, wash the filter cake, and dry to obtain 17.5 g of the fine compound of formula III, EE: 99.8%, and the yield is 79%.
[0032] The product in the above mother liquor was recovered to obtain 2 g of the refined compound of formula III, EE: 99.9%.
Embodiment 3
[0034] Add 30g of the compound represented by formula III to a 1L four-necked reaction flask, then add 490g of chloroform, control the temperature at 20-25°C and stir for 45 minutes, filter to remove the solid, and concentrate the mother liquor to dryness at 40°C under a pressure of -0.09MaP , to obtain the crude product of the compound shown in formula I.
[0035] The crude compound represented by formula I was added to 150 g of absolute ethanol, heated to 50-55° C. and stirred for 1 hour, then cooled to 20-25° C. for crystallization, and filtered to obtain the refined compound represented by formula I.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com