Fluorescent PCR kit for qualitative detection of HLA-B*1502 gene subtypes
A HLA-B, qualitative detection technology, applied in the direction of fluorescence/phosphorescence, microbial determination/inspection, biochemical equipment and methods, etc., to achieve the effect of accurate qualitative, simple steps, and reduce the risk of PCR product contamination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: the preparation of kit
[0033] 1. Design and synthesis of primers and probes
[0034]Using Primer Express 2.0, for exon 2 and exon 3 of HLA-B*1502 and the intron region in the middle, studies have shown that the current HLA-B genotyping is mainly concentrated in this region (Hughes AL, Nei M .Pattern of nucleotide substitution at MHC class I loci reveals overdominant selection. Nature, 1988, 335(6186): 167-170; Hughes AL, Yeager M. Natural selection at major histo-compatibility complex loci of vertebrats. Annu Rev Genet, 1998 , 32: 415-435; Gao X, Nelson GW, Karacki P, Martin MP, Phair J, Kaslow R, Goedert JJ, Buchbinder S, Hoots K, Vlahov D, O′Brien SJ, Carrington M. Effect of a single amino acid change in MHC class I molecules on the rate of progression-sion to AIDS.N Engl J Med, 2001, 344(22): 1668-1675; Xu Junping, Deng Zhihui, Zou Hongyan, etc., Chinese Han individuals HLA-A, - Determination of the full-length sequence of the B gene and the polymor...
Embodiment 2
[0068] Embodiment 2: the use of kit
[0069] 1. Sample detection
[0070] The positive control and negative control were respectively taken as DNA templates, and added to UNG enzyme, Taq polymerase, and fluorescent quantitative reaction solution containing specific PCR primers and specific fluorescent probes to form a PCR reaction system. In the positive control substance, the HLA-B*1502 plasmid standard substance (5.0 × 10 6 copy / microliter), as a standard for qualitative determination of samples.
[0071] The main components of the system are as follows:
[0072]
[0073] 2. Reaction procedure
[0074] Set the fluorescence detection channel for collecting FAM and VIC fluorescence signals, put the reaction tube into the fluorescence PCR instrument (ABI7500) to start amplification, the reaction procedure is as follows:
[0075] Table 3. PCR reaction program
[0076]
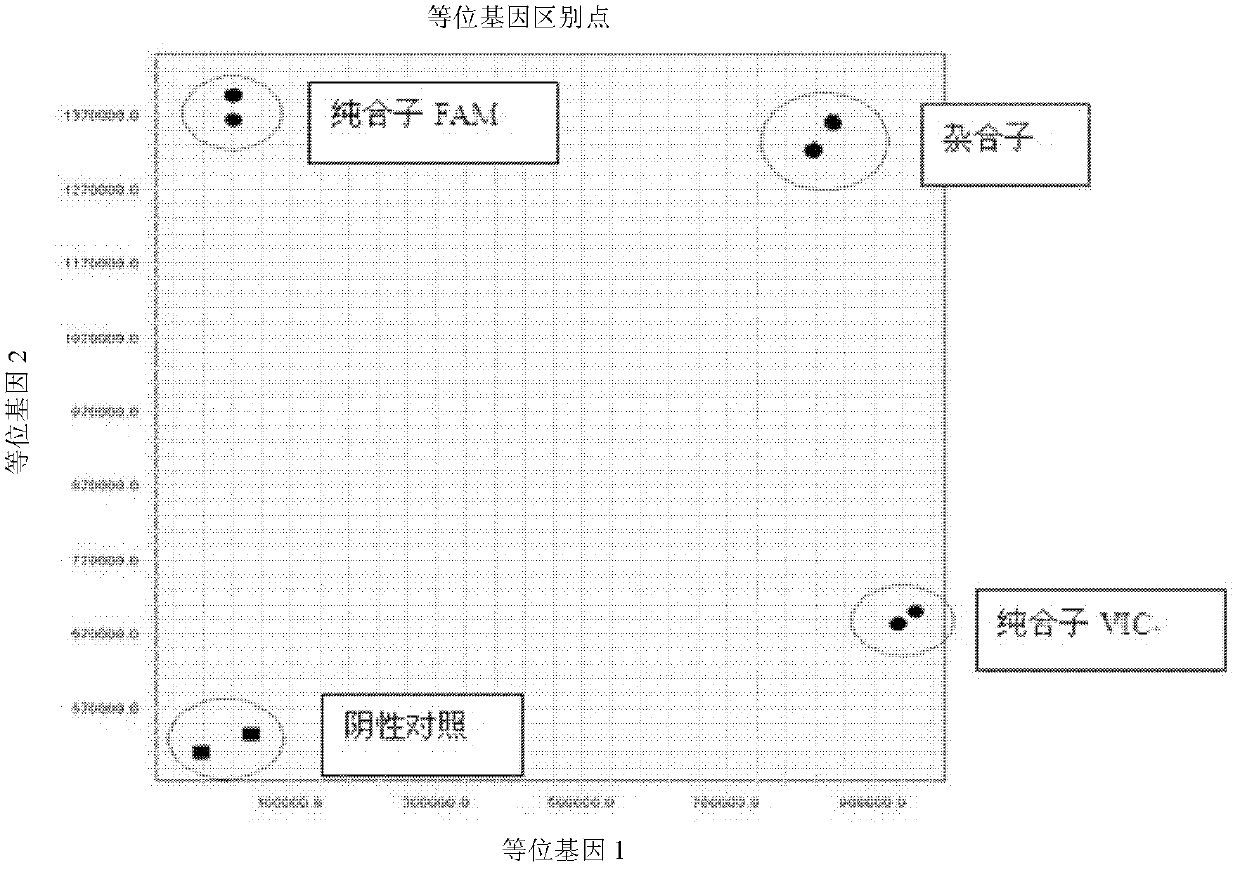
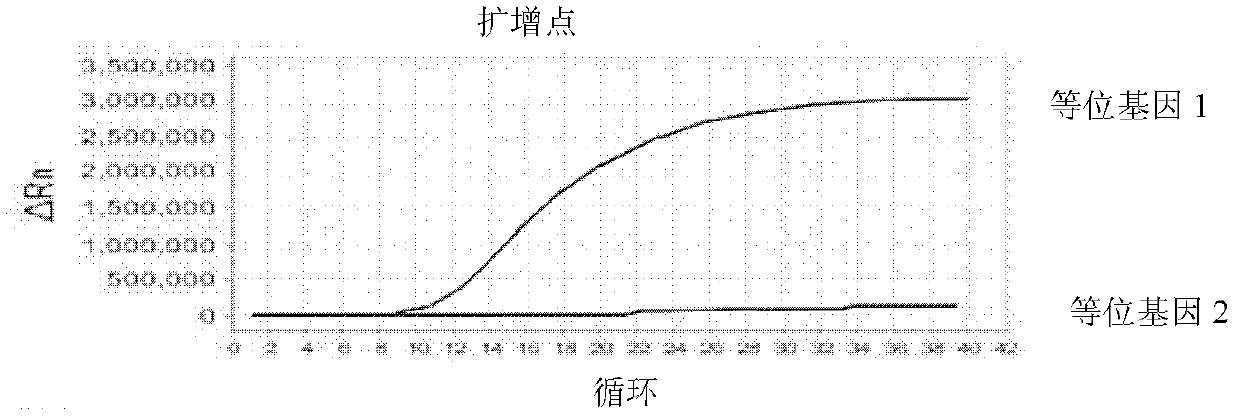
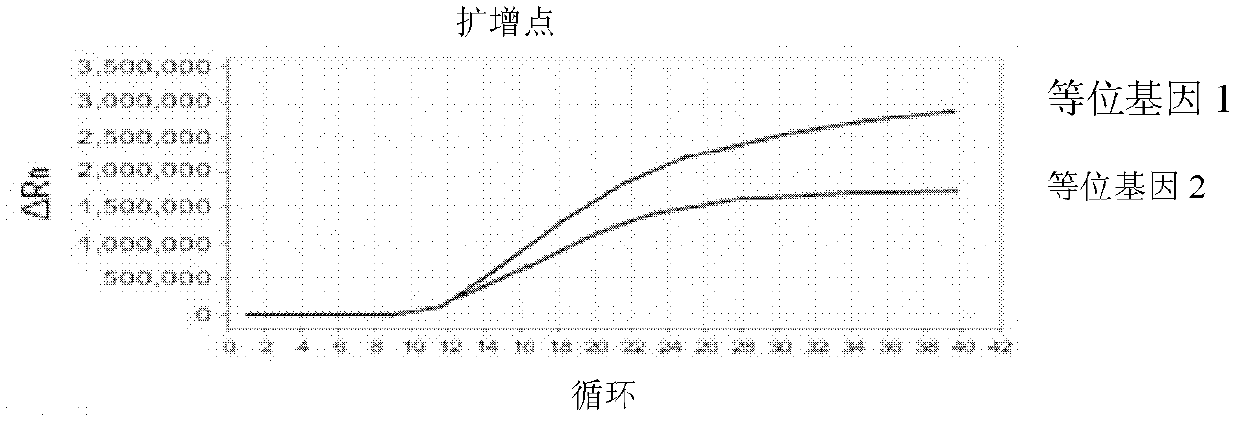
[0077] 3. Result judgment
[0078] The Ct value (cycle number) in the baseline range is 6-15 or au...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com