Lyophilized formulations for small modular immunopharmaceuticals
A technology of immune drugs and small modules, applied in drug combination, drug delivery, freeze-drying delivery, etc., can solve the problems of protein complexity and infeasibility of liquid preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0087] preparation of formulations
[0088] SMIP to be prepared TM Proteins can be produced using techniques well established in the art, including but not limited to recombinant techniques and peptide synthesis or a combination of these techniques. SMIP TM Proteins may be obtained from any in vivo or in vitro protein expression system, including but not limited to recombinant cells producing products, bacteria, fungal cells, insect cells, transgenic plants or plant cells, transgenic animals or animal cells or animal serum, ascites fluid, hybridomas or myeloma supernatant. Suitable bacterial cells include, but are not limited to, E. coli cells. Examples of suitable E. coli strains include: HB101, DH5[alpha], GM2929, JM 109, KW251, NM538, NM539 and any E. coli strain that cannot cleave foreign DNA. Suitable fungal host cells that may be used include, but are not limited to, Saccharomyces cerevisiae, Pichia pastoris, and Aspergillus cells. Suitable insect cells include, but...
Embodiment 1
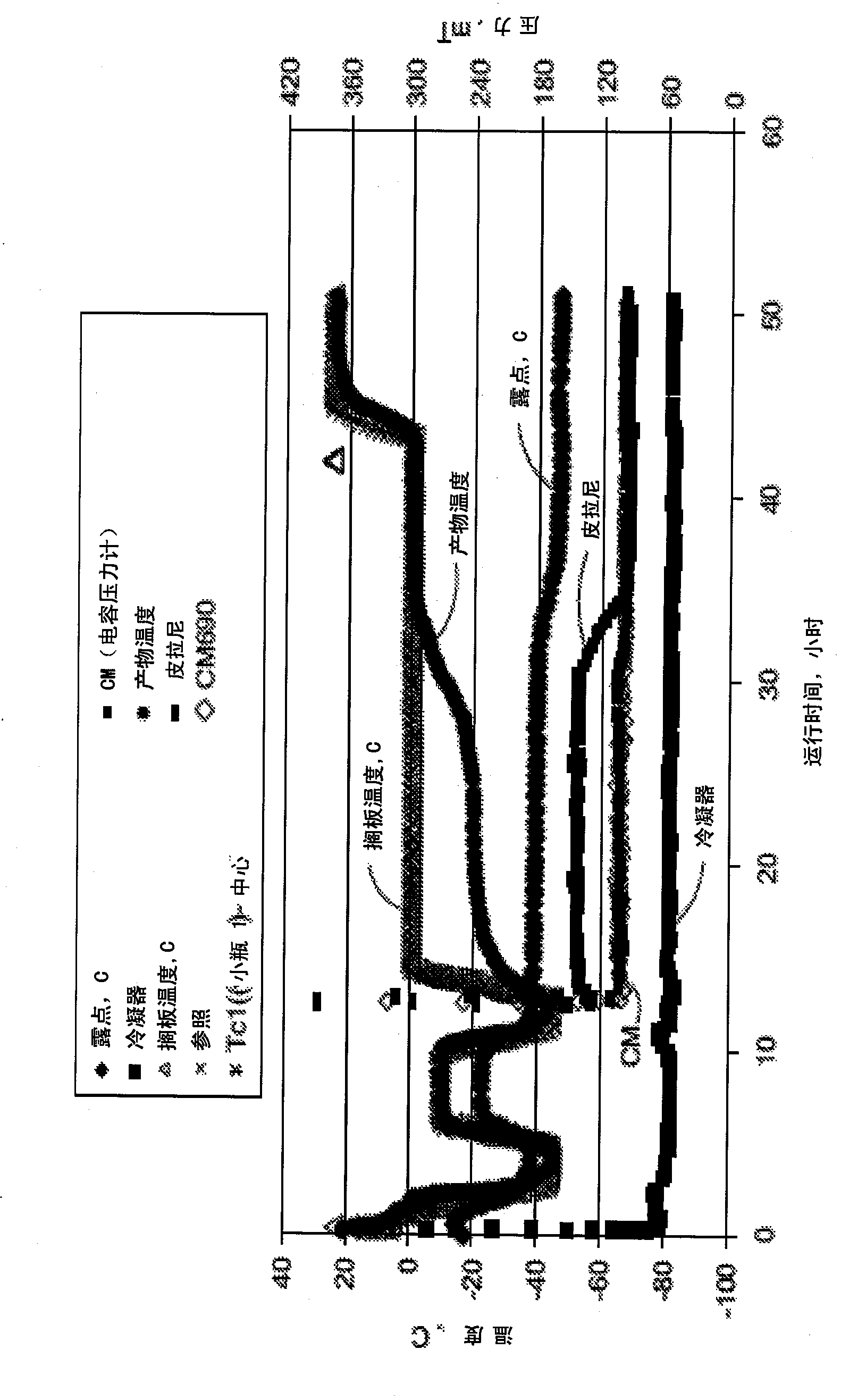
[0129] Example 1: Acetate-Glycine-Sucrose ("AGS") Lyophilized Formulation Containing 25 mg / ml TRU-015
[0130] In this example, the AGS formulation was designed to lyophilize TRU-015 at a concentration of approximately 25 mg / ml. Specifically, the AGS formulation used in this example included 6.5% sucrose, 50 mM glycine, 20 mM sodium acetate, pH 6.0. Protein concentration is 25 mg / ml, 100 mg protein per vial. Sucrose is used as a stabilizer and bulking agent, and glycine is added as a stabilizer and isotonic agent. Sodium acetate is a buffer. The AGS formulation had a glass transition of -34.2°C as measured by a modulated differential scanning calorimeter ("DSC"). The collapse temperature of the AGS formulation was found to be -31.4°C as measured by Freeze Drying Microscopy ("FDM"). The total lyophilization process in the laboratory scale lyophilizer lasted about 120 hours. An optional renaturation step at -10°C resulted in a shortened cycle time of 90 hours at laboratory ...
Embodiment 2
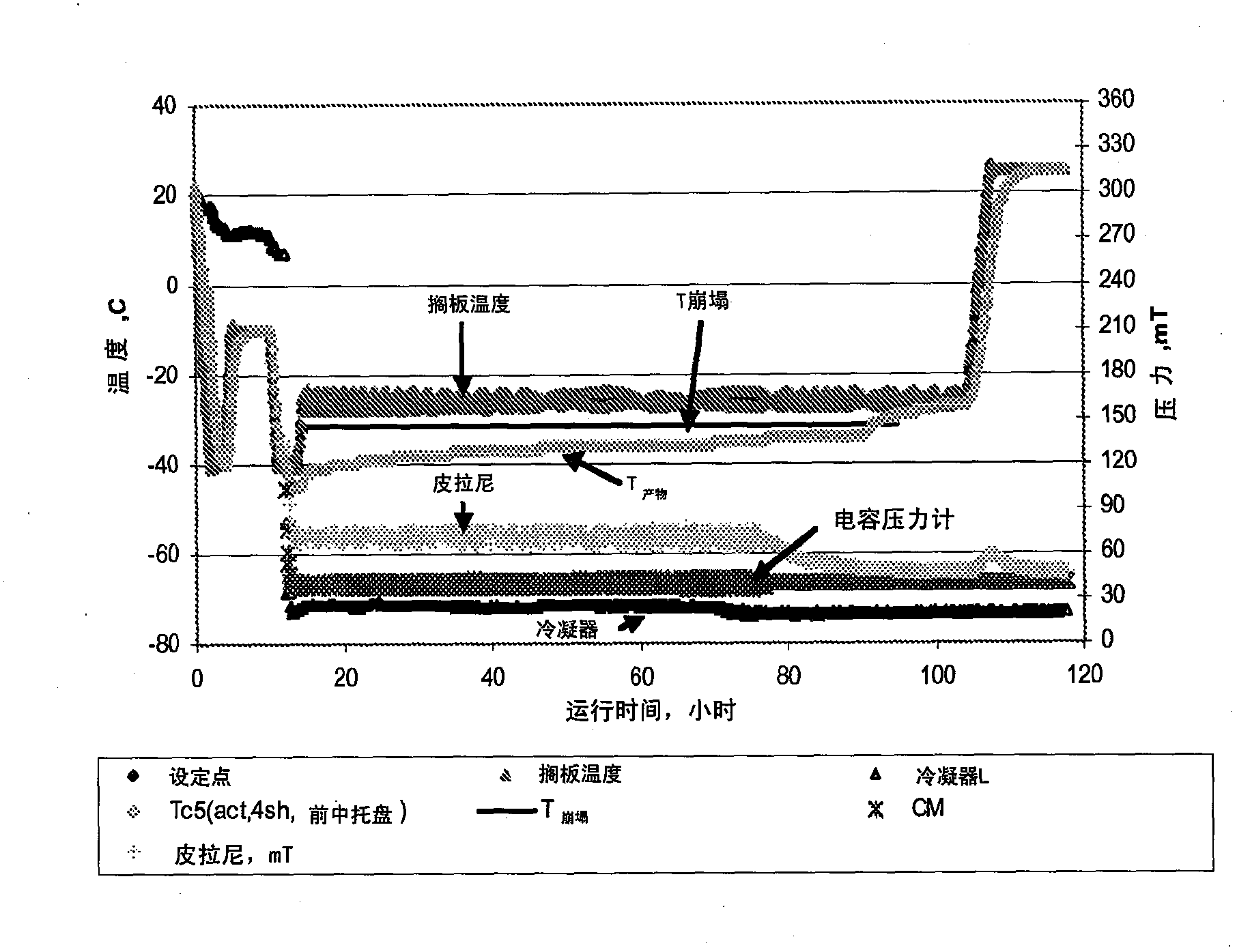
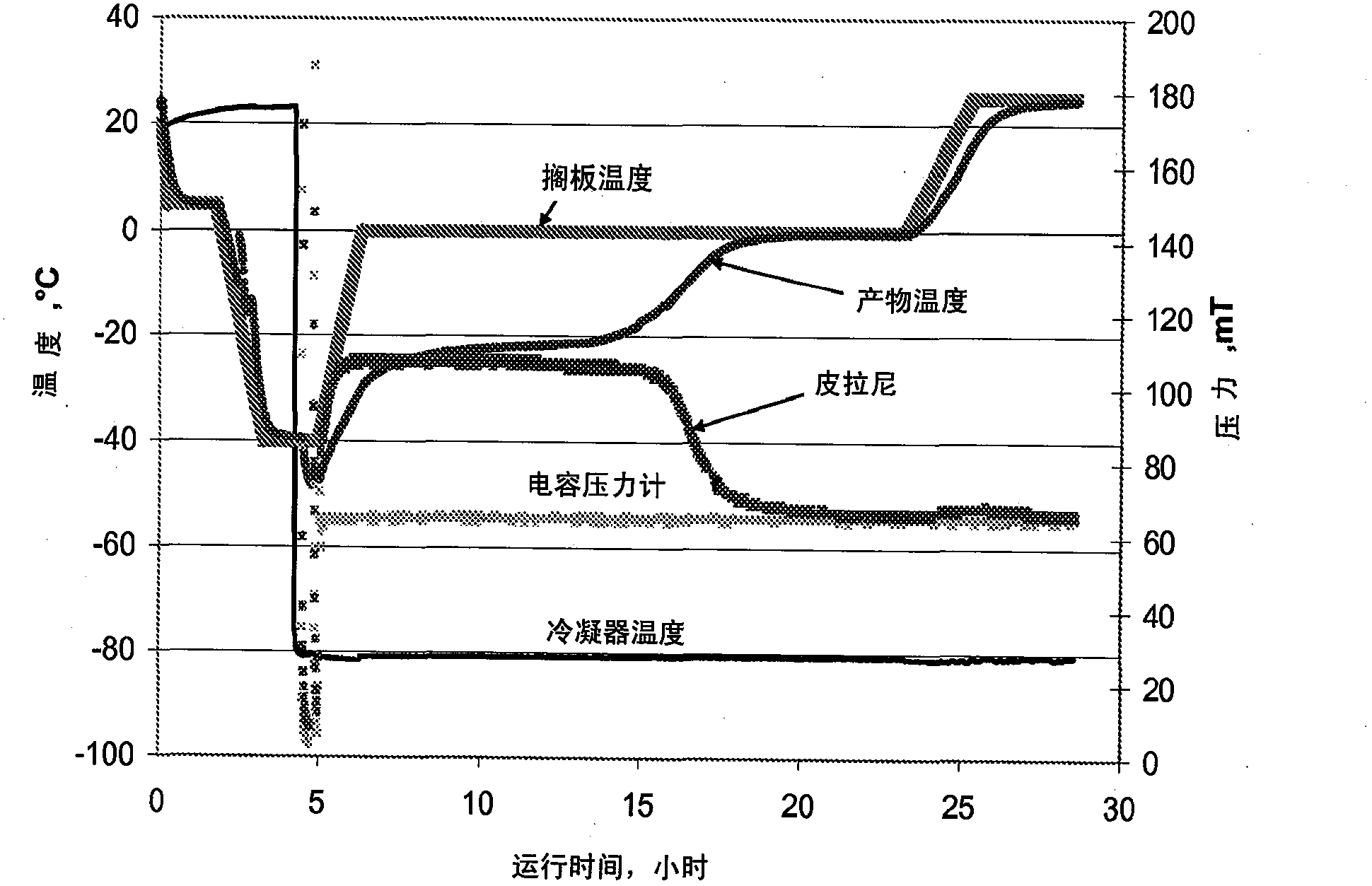
[0137] Example 2: Acetate-Mannitol-Sucrose ("AMS") or Histidine-Mannitol-Sucrose ("HMS") Formulations
[0138] In this example, two formulations were developed, AMS and HMS formulations. The acetate-mannitol-sucrose (AMS) based formulation contained 20 mM sodium acetate as a buffer, 4% mannitol as a bulking agent and 1% sucrose as a stabilizer. In the histidine-mannitol-sucrose (HMS) formulation, 20 mM histidine was used instead of sodium acetate buffer. The remaining components are the same (eg 4% mannitol, 1% sucrose). The solution pH was 6.0 for both formulations. The isotonicity of both formulations was 270 mOsm / kg. The fill volume for both formulations is 4 ml in 10-ml vials, with 100 mg protein per vial. A refolding step at -15°C was used during lyophilization. Without wishing to be bound by any theory, it is expected that this refolding step promotes crystallization of mannitol. For AMS and HMS formulations, once mannitol crystallizes, the glass transition tempera...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com