Method for producing 3-hydroxypropionic acid and other products
A technology of hydroxypropionic acid and acrylic acid, applied in the directions of biochemical equipment and methods, introduction of foreign genetic material, microorganisms, etc. using carriers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
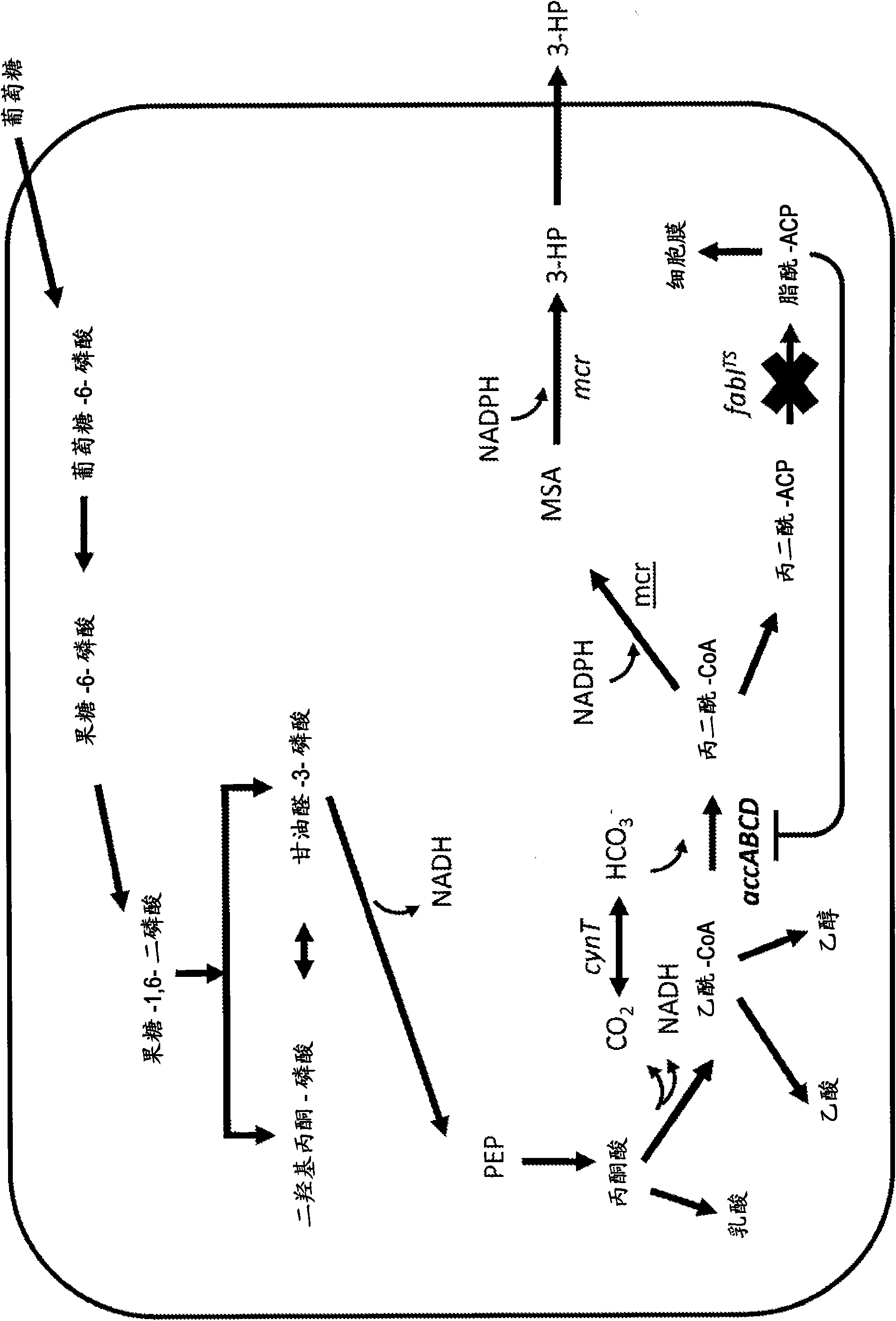
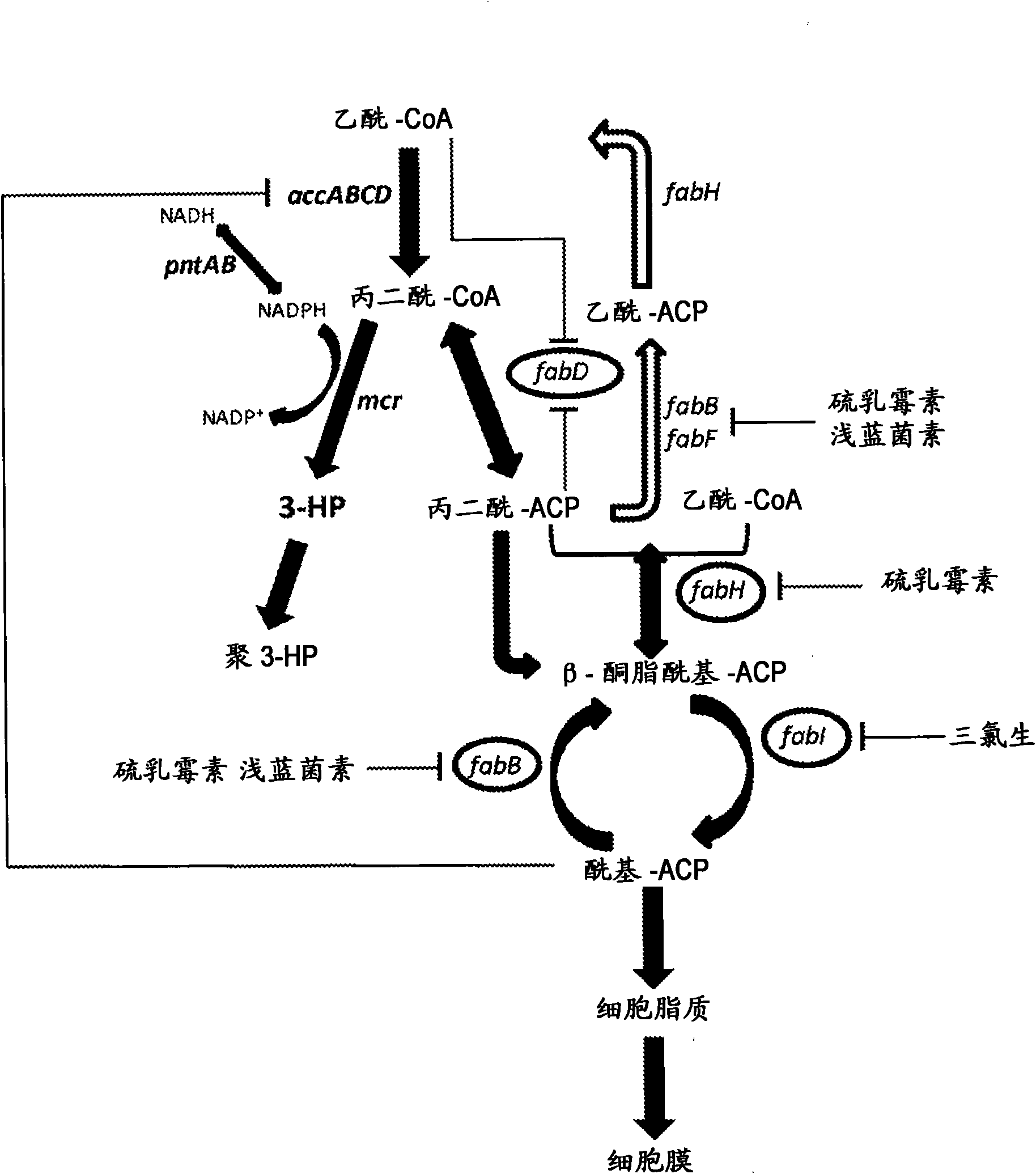
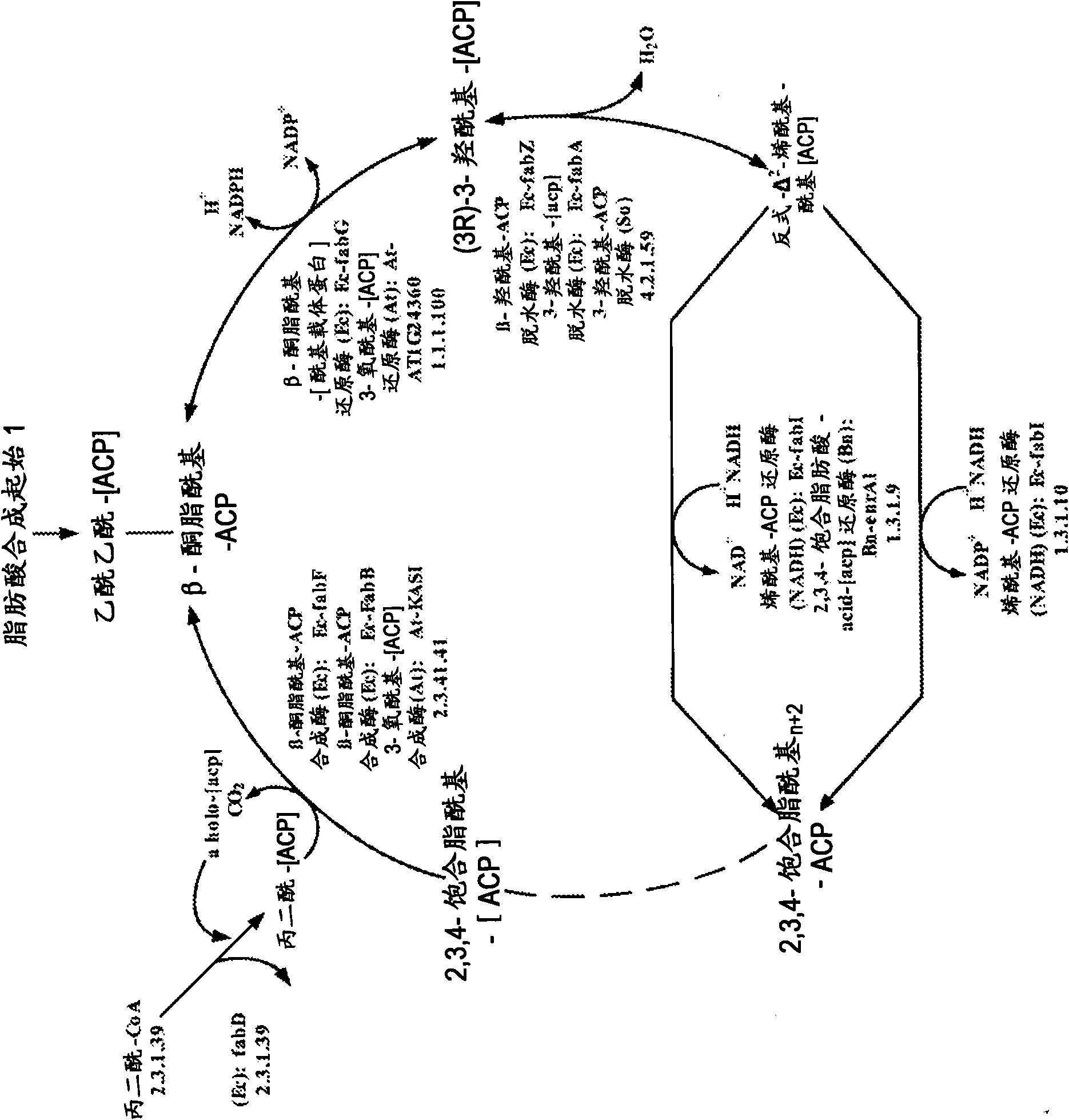
[0461] Example 1: Construction of a plasmid expressing malonyl-CoA reductase (mcr)
[0462] According to the service provided by the commercial DNA gene synthesis provider DNA2.0 (Menlo Park, CA USA), the nucleic acid sequence of the malonyl-CoA reductase gene from Chloroflexus aurantiacus was codon-optimized for E. coli. The gene sequence (SEQ ID NO: 803) integrates an EcoRI restriction site before its start codon and a HindIII restriction site afterwards. In addition, a ribosome binding site is preceded by the start codon. This gene construct was synthesized by DNA2.0 and provided in the pJ206 vector backbone (SEQ ID NO: 804). The plasmid DNA pJ206 containing the synthetic mcr gene was subjected to enzymatic restriction digestion using EcoRI and HindIII enzymes obtained from New England BioLabs (Ipswich, MA USA) according to the manufacturer's instructions. The digestion mixture was separated by agarose gel electrophoresis and the appropriate DNA fragments were recovered as d...
Embodiment 2
[0466] Example 2: Construction of a plasmid expressing transhydrogenase (pntAB)
[0467] The non-inducer-dependent E. coli promoter derived from the tpiA gene (P tpiA ) Fusion with the pyridine nucleotide transhydrogenase gene pntAB (SEQ ID NO: 779 and SEQ ID NO: 781) was produced by amplifying the tpiA promoter region and pntAB region from genomic E. coli K12 DNA using polymerase chain reaction. For the pntAB gene, the pntAB forward primer GGGAACCATGGCAATTGGCATACCAAG (SEQ ID NO: 807, note that all the primers disclosed herein are artificial sequences) and the pntAB reverse primer containing the NcoI site of the starting Met incorporated into the pntA protein sequence were used GGGTTACAGAGCTTTCAGGATTGCATCC (SEQ ID NO: 808) amplified this region. Similarly, the P tpiA The region was amplified using the forward primer GGGAACGGCGGGGAAAAACAAACGTT (SEQ ID NO: 809) and the reverse primer GGTCCATGGTAATTCTCCACGCTTATAAGC (SEQ ID NO: 810) containing the NcoI restriction site. The polyme...
Embodiment 3
[0471] Example 3: Construction of a plasmid expressing acetyl-CoA carboxylase (accABCD)
[0472] A plasmid carrying two operons that can express the components of the E. coli acetyl-CoA carboxyltransferase complex was constructed by a commercial DNA gene synthesis provider DNA2.0 (Menlo Park, CA USA). This construct integrates the DNA sequences of accA and accD genes under the control of an inducer-independent promoter derived from the E. coli tpiA gene, and under the control of an inducer-independent promoter derived from the E. coli rpiA gene The DNA sequences of accB and accC genes below. Each coding sequence has a ribosome binding sequence before it. The designed operon was provided in the pJ251 vector backbone and was named pJ251:26385 (SEQ ID NO:817).
[0473] The tpiA promoter of the pJ251:26385 plasmid was modified to provide higher expression. The modification was introduced by amplifying the plasmid using the forward primer GCGGGGCAGGAGGAAAAACATG (SEQ ID NO: 818) and t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com