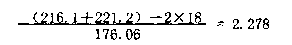Sodium hyaluronate eye drops and a preparation method thereof
A technology of sodium hyaluronate and eye drops, which is applied in the field of medicine, can solve the problems of easy contamination and growth of bacteria, and achieve the effects of stable and feasible preparation process, prolonging tear film breakup time and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
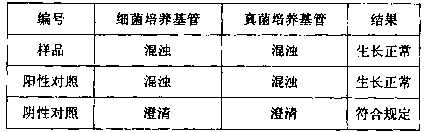
Examples
Embodiment 1
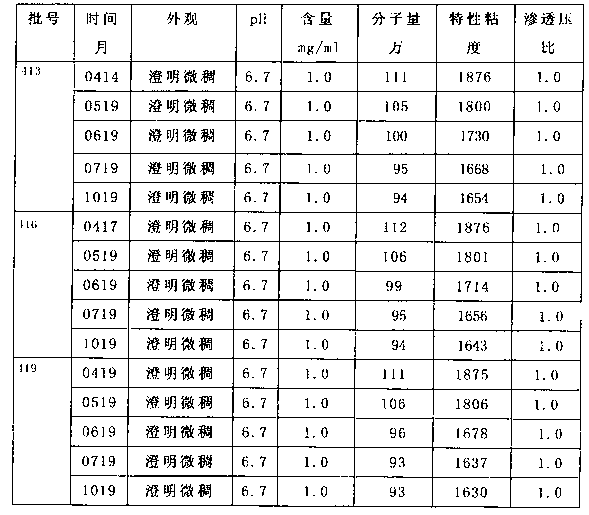
[0068] Sodium hyaluronate eye drops, each 100ml consists of the following components:
[0069] Sodium Hyaluronate 100mg, Boric Acid 150mg,
[0070] Borax 12mg, Edetate Disodium 10mg,
[0071] 6-aminocaproic acid 100mg, mannitol 100mg,
[0072] Sodium Chloride 700mg, Benzalkonium Chloride 5mg,
[0073] Borneol 10mg, add water for injection to 100ml.
[0074] The preparation method of above-mentioned sodium hyaluronate eye drops, comprises the following steps:
[0075] 1) Take 50% of the prepared water for injection in advance and cool it to 50~60°C and pour it into the mixing bucket, accurately weigh the sodium hyaluronate, add the sodium hyaluronate while stirring, soak until completely dissolved;
[0076] 2) Take 30% of the prepared water for injection and put it in a stainless steel bucket, accurately weigh boric acid, borax, disodium edetate, 6-aminocaproic acid, mannitol, and sodium chloride, and add them into the stainless steel bucket in sequence , after stirring an...
Embodiment 2
[0084] Sodium hyaluronate eye drops, each 100ml consists of the following components:
[0085] Sodium Hyaluronate 90mg, Boric Acid 110mg,
[0086] Borax 12mg, Edetate Disodium 10mg,
[0087] 6-aminocaproic acid 100mg, mannitol 90mg,
[0088] Sodium Chloride 700mg, Benzalkonium Chloride 5mg,
[0089] Borneol 10mg, add water for injection to 100ml.
[0090] The preparation method is shown in Example 1.
Embodiment 3
[0092] Sodium hyaluronate eye drops, each 100ml consists of the following components:
[0093] Sodium Hyaluronate 140mg, Boric Acid 180mg,
[0094] Borax 18mg, Edetate Disodium 12mg,
[0095] 6-aminocaproic acid 150mg, mannitol 140mg,
[0096] Sodium Chloride 650mg, Benzalkonium Chloride 5mg,
[0097] Borneol 12mg, add water for injection to 100ml.
[0098] The preparation method is shown in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com