New compound m-hydroxyphenyl tetrazine dicarbonamide, preparation and application thereof
A technology of hydroxyphenyl and dicarboxamide, which is applied to the new compound m-hydroxyphenyltetrazine dicarboxamide and the fields of preparation and application, can solve the problems of no antitumor activity and the like, and achieves easy availability of raw materials, simple preparation method, The effect of low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
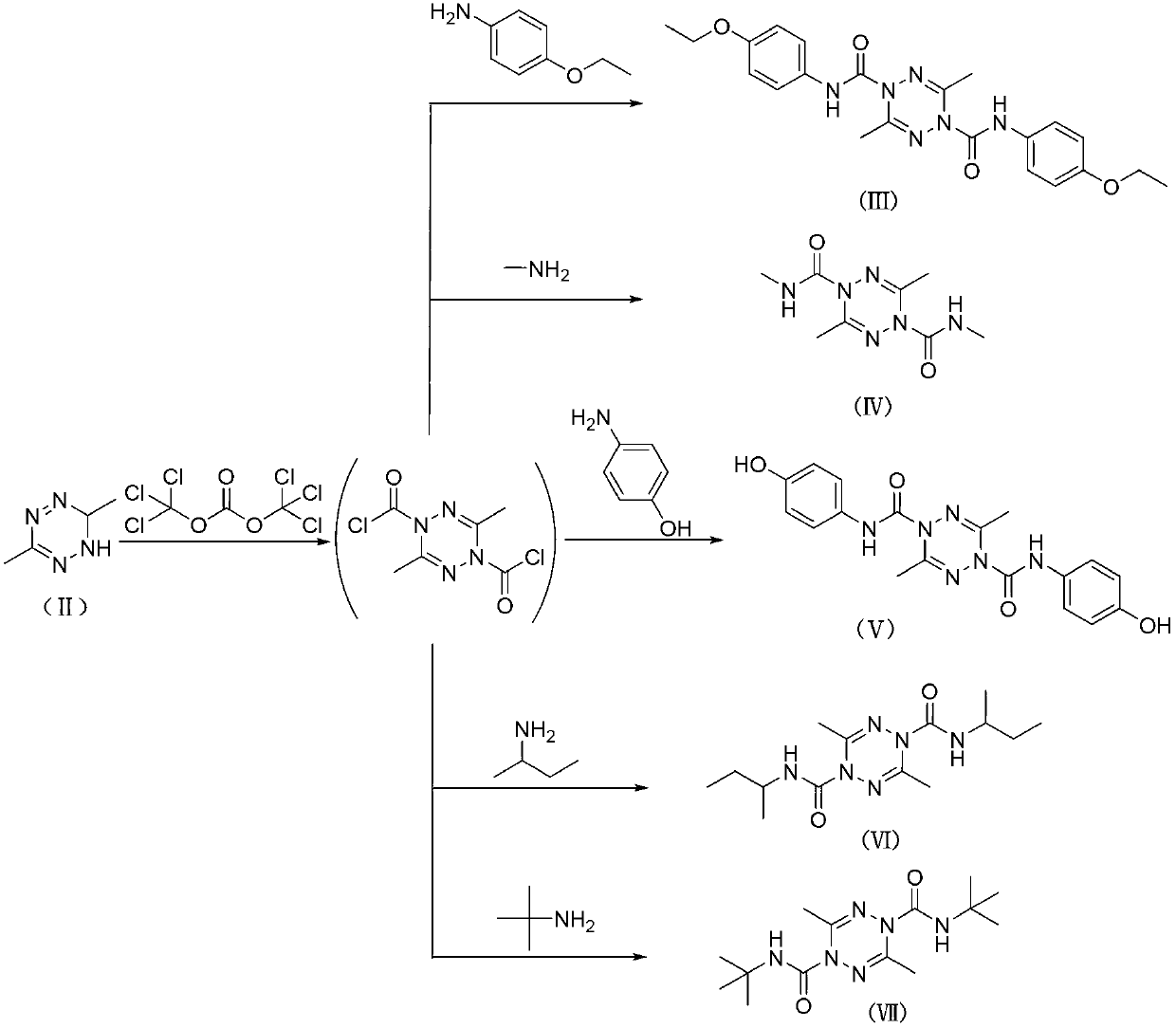
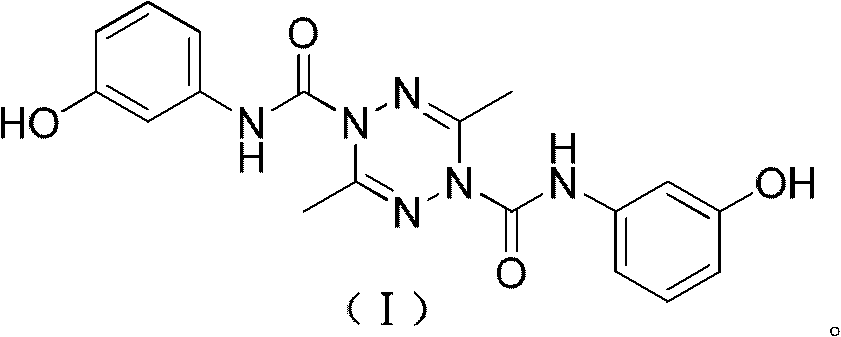
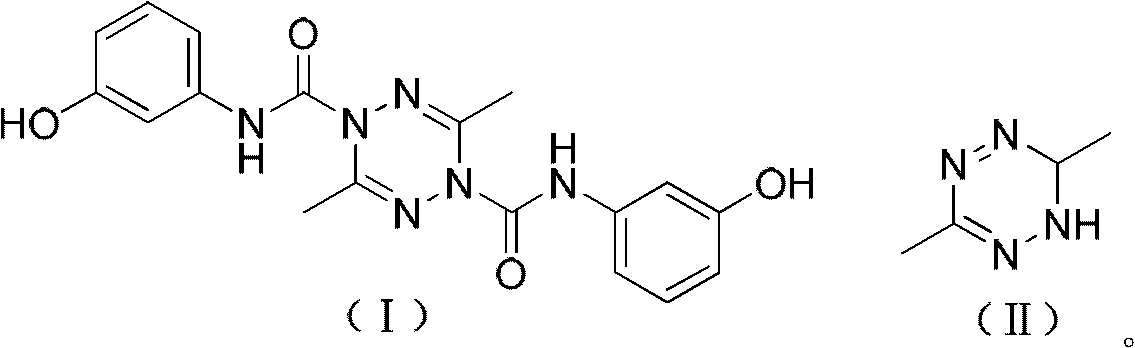
[0024] Example 1: N 1 , N 4 - Preparation of two (3-hydroxyphenyl)-3,6-dimethyl-1,2,4,5-tetrazine-1,4-dicarboxamide (I)
[0025] Dissolve 2.00 grams (17.8 mmol) of 3,6-dimethyl-1,6-dihydro-1,2,4,5-tetrazine (II) and 2.16 grams (17.8 mmol) of N,N-xylidine In 50 milliliters of dichloromethane solution, prepare a preliminary solution (that is, a solution containing 3,6-dimethyl-1,6-dihydro-1,2,4,5-tetrazine and a basic catalyst), 250 milliliters 10.60 g (35.7 mmol) of bis(trichloromethyl)carbonate and 20 ml of dichloromethane were sequentially added into the three-necked flask, stirred at 0° C., and the preliminary solution was slowly added dropwise. After the addition was completed within 10 minutes, TLC followed and detected, and the reaction was refluxed for 4 hours. Then the temperature was lowered to 5° C., nitrogen gas was blown, and a solution obtained by dissolving 3.90 g (35.7 mmol) of m-hydroxyaniline in 20 ml of dichloromethane was added dropwise. After dripping, T...
Embodiment 2
[0026] Example 2: N 1 , N 4 - Preparation of two (3-hydroxyphenyl)-3,6-dimethyl-1,2,4,5-tetrazine-1,4-dicarboxamide (I)
[0027] Dissolve 2.00 g (17.8 mmol) of 3,6-dimethyl-1,6-dihydro-1,2,4,5-tetrazine (II) and 0.98 g (12.4 mmol) of pyridine in 10 ml of methanol to prepare To form a preparatory solution, 3.54 g (11.9 mmol) of bis(trichloromethyl)carbonate and 10 ml of methanol were sequentially added to a 50 ml three-necked flask, stirred at -5°C, and the preparatory solution was slowly added dropwise. After the addition was completed within 10 minutes, TLC followed and detected, and the reaction was refluxed for 10 hours. Then the temperature was lowered to 6° C., nitrogen gas was blown, and a solution obtained by dissolving 5.84 g (53.5 mmol) of m-hydroxyaniline in 6 ml of ethanol was added dropwise. After dripping, TLC tracking detection, heating and reflux for 27 hours, distilled off methanol and ethanol, added 20 ml of chloroform to the residue, stirred and heated, re...
Embodiment 3
[0028] Example 3: N 1 , N 4 - Preparation of two (3-hydroxyphenyl)-3,6-dimethyl-1,2,4,5-tetrazine-1,4-dicarboxamide (I)
[0029] Dissolve 2.00 g (17.8 mmol) of 3,6-dimethyl-1,6-dihydro-1,2,4,5-tetrazine (II) and 0.91 g (7.4 mmol) of 4-dimethylaminopyridine in Prepare a preparatory solution in 40 ml of chloroform solution, add 4.30 g (14.5 mmol) of bis(trichloromethyl)carbonate and 20 ml of chloroform to a 100 ml three-necked flask successively, stir at -10°C, and slowly add the preparatory solution dropwise. After the addition was completed within 40 minutes, TLC followed and detected, and the reaction was refluxed for 8 hours. Then the temperature was lowered to 6° C., nitrogen gas was blown, and a solution obtained by dissolving 7.77 g (71.2 mmol) of m-hydroxyaniline in 10 ml of chloroform was added dropwise. After dripping, TLC tracking detection, heated to reflux for 12 hours, evaporated chloroform, and the residue was recrystallized with 20 milliliters of ethanol. Othe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com