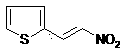
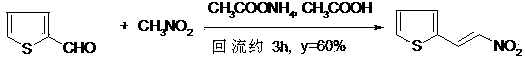Synthesis method of 2-nitroethenyl thiophene
A technology of nitroethylene and synthesis method, which is applied in the direction of organic chemistry, can solve the problems of large amount of nitromethane and high purification cost, and achieve the effects of increasing yield, shortening reaction time, and promoting reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Put 11.2g (0.1mol) 2-thiophene carboxaldehyde, 7.3g (0.12mol) nitromethane, 4ml acetonitrile solution of 0.9g (0.01mol) β-alanine in the reaction bottle, 60ml N,N-di Methylformamide, 90°C ultrasonic radiation (100W, 40kHz) for 1h, cooled, distilled off acetonitrile, filtered, concentrated, the residue was recrystallized to give 14.6g of 2-nitroethylenethiophene (yield 94%), yellow crystals, m .p79~80℃, content 99.2% (GC). β-alanine recovery 0.75g, can be reused.
Embodiment 2
[0032] Put 11.2g (0.1mol) 2-thiophenecarbaldehyde, 6.1g (0.10mol) nitromethane, 4ml acetonitrile solution of 0.9g (0.01mol) β-alanine in the reaction flask, 60ml N,N-di Methylformamide, 90°C ultrasonic radiation (100W, 40kHz) for 1h, cooled, distilled off acetonitrile, filtered, concentrated, recrystallized to give 13.3g of 2-nitrovinylthiophene (yield 86%), yellow crystal, m.p 79 ~80°C, content 99.2% (GC). β-alanine recovery 0.75g, can be reused.
Embodiment 3
[0034] Put 11.2g (0.1mol) 2-thiophene carboxaldehyde, 8.5g (0.14mol) nitromethane, 4ml acetonitrile solution of 0.9g (0.01mol) β-alanine in the reaction bottle, 60ml N,N-di Methylformamide, 90°C ultrasonic radiation (100W, 40kHz) for 1h, cooled, distilled off acetonitrile, filtered, concentrated, and recrystallized to give 14.7g of 2-nitroethylenethiophene (yield 95%), yellow crystal, m.p 79 ~80°C, content 99.1% (GC). 0.74g of β-alanine is recovered and can be reused.
[0035] In the above examples, the method of the present invention adds 2-thiophene formaldehyde, nitromethane, β-alanine solution dissolved in acetonitrile, and solvent into the reaction flask, and reacts with ultrasonic radiation (100W, 40kHz) at 90°C for 1 hour, and after post-treatment, the The product 2-nitroethylenethiophene was obtained, and the recovery of β-alanine was more than 82%, which could be reused. Compared with the existing technology, the process has the characteristics of simple operation, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com