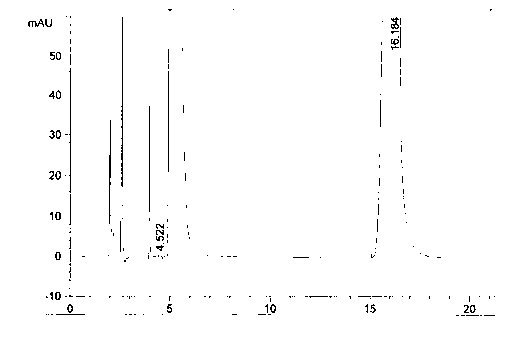
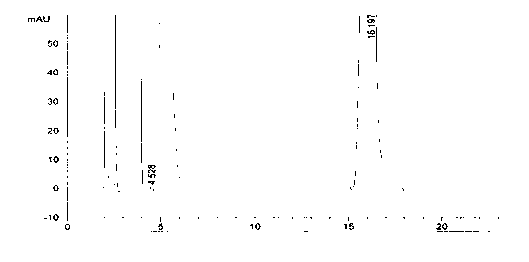
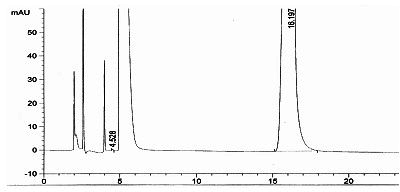
Preparation method of azacitidine
A technology for azacitidine and a compound is applied in the field of preparation of azacitidine, which can solve the problems of increased impurities, unfavorable purification, difficult operation, etc., and achieves reduction of hydrolysis side reactions and generation of impurities, reduction of solvent usage, and improved performance. The effect of reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Preparation of Compound I: Hexamethyldisilane (5600 mL), 5-azacytosine (800 g, 7.14 mol) and ammonium sulfate (34 g) were put into a dry 20 L reaction pot. Turn on the stirring, stir well, heat to 140-142°C and reflux for about 1 hour, the system gradually changes from cloudy to clear. Continue to keep the temperature at 140-142° C. for reflux reaction for 15 hours. Naturally cool to 75-80°C and turn on the vacuum pump, transfer the material to the distillation bottle of the rotary evaporator, and carry out vacuum distillation. In addition to hexamethyldisilazane. The obtained viscous liquid was compound Ⅰ (1687g, 7mol).
[0036] Preparation of compound Ⅱ: Add tetraacetyl ribose (2720g, 8.55mol), mix well, vacuumize, control the vacuum degree -0.09MPa, and slowly heat up the oil bath to 160-170°C until the reaction material melts, and continue the reaction under this condition After 1.5-2.5 hours, it was cooled to 20-25°C at room temperature to obtain 2518 g (6.8 mol...
Embodiment 2
[0041] The same as Example 1, the difference is the preparation of compound II: add tetraacetyl ribose (3342g, 10.5mol), mix well, vacuumize and feed nitrogen, control the vacuum degree -0.1MPa, and slowly raise the temperature of the oil bath to 170-180 ℃ until the reaction material melted, reacted under this condition for 2.5-3.5 hours, then cooled in vacuum for 0.1-0.5h to 30-40 ℃ to obtain 2599g (7.02mol) of compound II as a foamy solid.
[0042]
Embodiment 3
[0044] The same as Example 1, the difference is the preparation of compound II: add tetraacetyl ribose (1781g, 5.6mol), mix well, vacuumize, control the vacuum degree -0.01MPa, and slowly heat up the oil bath to 145-150°C until the reaction material Melted, reacted under this condition for 3.5 to 4.5 hours, then cooled to obtain 2407 g (6.5 mol) of compound II as a foamy solid.
[0045]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com