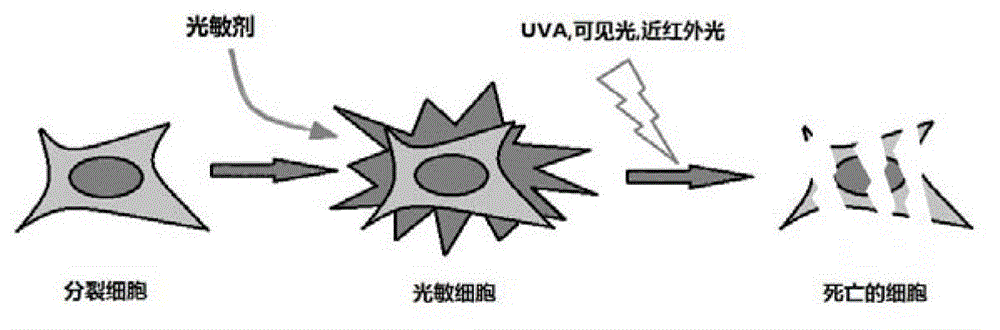
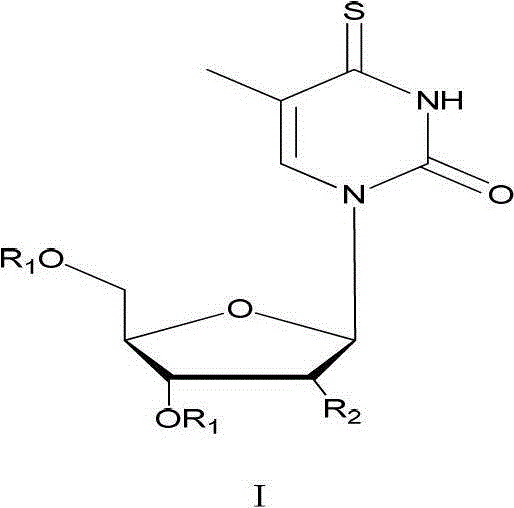
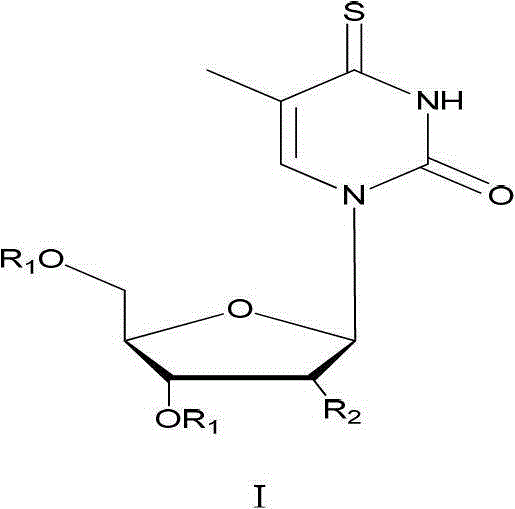
Synthetic method of 4-sulfur thymidine and analogues of 4-sulfur thymidine under microwave irradiation
A sulfur-thymidine and microwave-assisted technology, which is applied in the field of pharmaceutical chemistry and pharmaceuticals, can solve the problems of unfavorable anti-cancer active drug research, time-consuming and energy-consuming, low yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: A method for synthesizing 4-thiothymidine, including the following steps:
[0035] (1) Synthesize 3',5'-O-dioxoacetylthymidine (Ⅲ) by acetylation to protect the hydroxyl group on the sugar ring.
[0036]
[0037] The compound thymidine (1.00g, 3.87mmol) (II) was dissolved in anhydrous pyridine (40.4ml, 502mmol), cooled to 0°C, and anhydrous acetic anhydride (8.3ml, 87.8mmol) was added at 0°C. Continue stirring for 5h (TLC tracking). After taking it out, the solvent was removed under reduced pressure and recrystallized from methanol to obtain white massive crystals (III) (2.41g, 9.33mmol), with a yield of 95%, mp 129-131°C. 1 H NMR(400MHz, DMSO-d 6 )δ: 11.39 (br s, 1H, NH), 7.50 (s, 1H, 6-H), 6.18 (t, J = 8.0 Hz, 1H, 1'-H), 5.18 (t, J = 4.0 Hz, 1H,3'-H), 4.25(d,J=4.0Hz,2H,5'-H), 4.14(d,J=4Hz,1H,4'-H,),2.25-2.48(m,2H, 2'-H),2.07(s,6H,2×-OC=OCH 3 ),1.80(s,3H,-CH 3 ).
[0038] (2) Synthesis of 3',5'-O-dioxoacetyl-4-thiothymidine (IV) by replacing oxygen with sulfur b...
Embodiment 2
[0044] Example 2: The effect of microwave reaction time on the thiosulfate yield in step B
[0045] (1) Synthesis of 3',5'-O-dioxoacetyl thymidine by acetylation of thymidine to protect the hydroxyl group on the sugar ring. For specific examples, refer to step (1) in Example 1.
[0046] (2) Synthesis of 3',5'-O-dioxoacetyl-4-thiothymidine by using the microwave method to substitute sulfur for oxygen.
[0047] The compound 3',5'-O-dioxoacetyl-4-thiothymidine (1.0g, 3.06mmol) was dissolved in anhydrous dioxane (55ml, 643mmol), and P was added 2 S 5 (1.02g, 4.59mmol), put into a microwave reactor, irradiate at 800W power for 5min (TLC tracking), repeat time, microwave reaction time are 8min, 10min, 15min, respectively, the yield under different microwave reaction time is shown in Table 1. It can be seen from the table that the microwave reaction time is 8-10min with the highest yield.
[0048] Table 1 The influence of microwave reaction time on product yield
[0049] Time / min
[0050]...
Embodiment 3
[0052] (1) Reacting thymidine with benzoyl chloride to protect the hydroxyl group on the sugar ring. For specific examples, refer to the literature Fox et al., JACS, 1959, vol. 81, p. 178-187. The 3',5'-O-dibenzoyl thymidine was obtained, the yield was 85%, and the NMR data was consistent with the literature value.
[0053] (2) Using microwave method to synthesize 3',5'-O-dibenzoyl-4-thiothymidine by replacing oxygen with sulfur.
[0054] The compound 3’,5’-O-dibenzoylthymidine (1.38g, 3.06mmol) was dissolved in anhydrous dioxane (55ml, 643mmol), and P 2 S 5 (1.02g, 4.59mmol), put into a microwave reactor, irradiate for 8min under 800W power (TLC tracking). After the reaction was stopped, the filtrate was collected and the solvent was removed under reduced pressure to obtain a crude product. The crude product was recrystallized from ethanol to obtain 1.20 g of a yellow solid with a yield of 83.9%. Melting point: 161-162°C. Anal.Calcd.for C 10 H 16 N 3 O 4 Cl:C,43.24;H,5.81;N,15.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com