Method and device for deeply removing copper of nickel thiocarbonate from nickel-containing solution
A technology of thiocarbonic acid and nickel solution, which is applied in the field of copper-nickel hydrometallurgy, can solve the problems of short reaction time and failure to meet the requirements of copper removal, and achieve the goals of reducing labor intensity, simple copper removal operation process, and reducing heat loss Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
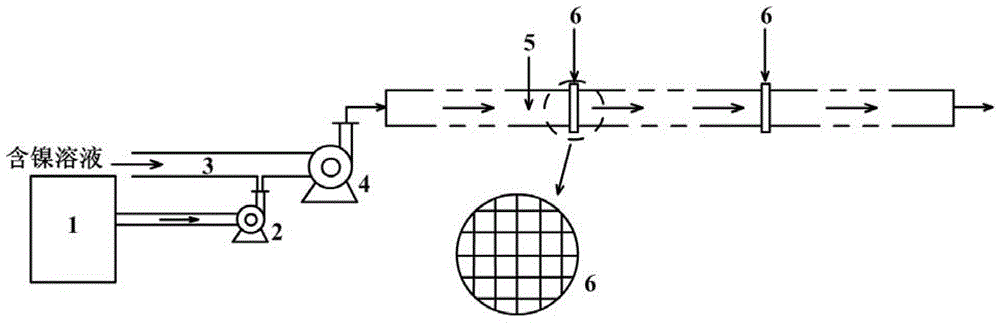
[0027] First adjust the pH value of the nickel electrolytic anolyte with a nickel concentration of 70g / l to 4.0, and then mix it with Na 2 CS 3 The solution was mixed and reacted for 10 minutes to obtain a nickel thiocarbonate slurry with a thiocarbonate concentration of 0.5 mol / l as a copper removal agent; then the pH of the nickel electrolytic anolyte with a copper concentration of 0.5 g / l was adjusted to 4.5, and the temperature was heated to 60°C; then mix according to the molar ratio of thiocarbonate in the copper removal agent to copper in the nickel electrolysis anolyte is 1.3, and control the feeding speed of the nickel thiocarbonate slurry feed pump to 1.56m 3 / h, inject it into the nickel-containing solution feed pipe, and mix it with the nickel electrolysis anolyte at 76.56m 3 / h feed rate [that is, the flow rate of the mixed solution V (m 3 / h)] was injected into a pipe reactor with a diameter of 40cm and a length of 305m through a nickel-containing solution feed...
Embodiment 2
[0029] First adjust the pH value of the nickel sulfate solution with a nickel concentration of 70g / l to 5.0, and then mix it with Na 2 CS 3 The solution was mixed and reacted for 20 minutes to obtain a nickel thiocarbonate slurry with a thiocarbonate concentration of 1.2mol / l as a copper removal agent; then the pH of the nickel electrolytic anolyte with a copper concentration of 0.6g / l was adjusted to 5.0, and the temperature was heated to 65°C; then mix according to the molar ratio of thiocarbonate in the copper removal agent to copper in the nickel electrolysis anolyte as 1.4, and control the feeding speed of the nickel thiocarbonate slurry feed pump to 0.82m 3 / h, inject it into the nickel-containing solution feed pipe, and mix it with the nickel electrolysis anolyte at 75.82m 3 / h feed rate [that is, the flow rate of the mixed solution V (m 3 / h)] was injected into a pipe reactor with a diameter of 30cm and a length of 268m through a nickel-containing solution feed pump,...
Embodiment 3
[0031] First adjust the pH value of the nickel electrolytic anolyte with a nickel concentration of 70g / l to 5.0, and then mix it with Na 2 C 2 S 5 The solution was mixed and reacted for 15 minutes to obtain a nickel thiocarbonate slurry with a thiocarbonate concentration of 0.8mol / l as a copper removal agent; then the pH of the nickel electrolytic anolyte with a copper concentration of 0.5g / l was adjusted to 4.8, and the temperature was heated to 60°C; then mix according to the molar ratio of thiocarbonate in the copper removal agent to copper in the nickel electrolysis anolyte as 1.2, and control the feeding speed of the nickel thiocarbonate slurry feed pump to 0.82m 3 / h, inject it into the nickel-containing solution feed pipe, and mix it with the nickel electrolysis anolyte at 75.82m 3 / h feed rate [that is, the flow rate of the mixed solution V (m 3 / h)] was injected into a pipe reactor with a diameter of 35cm and a length of 329m through a nickel-containing solution fe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com