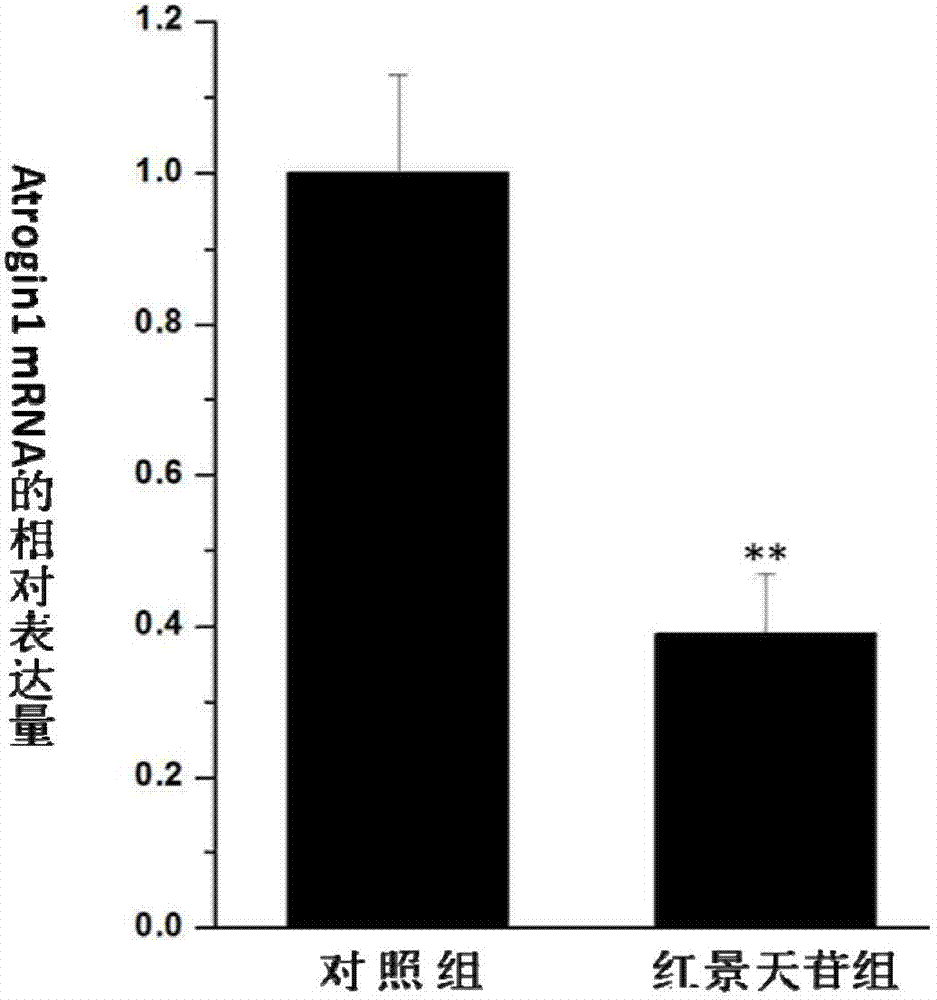
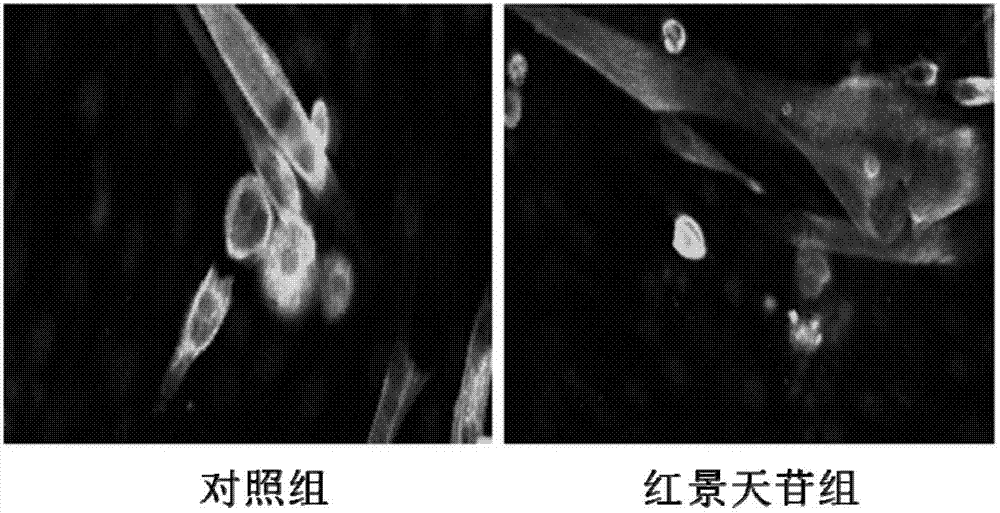
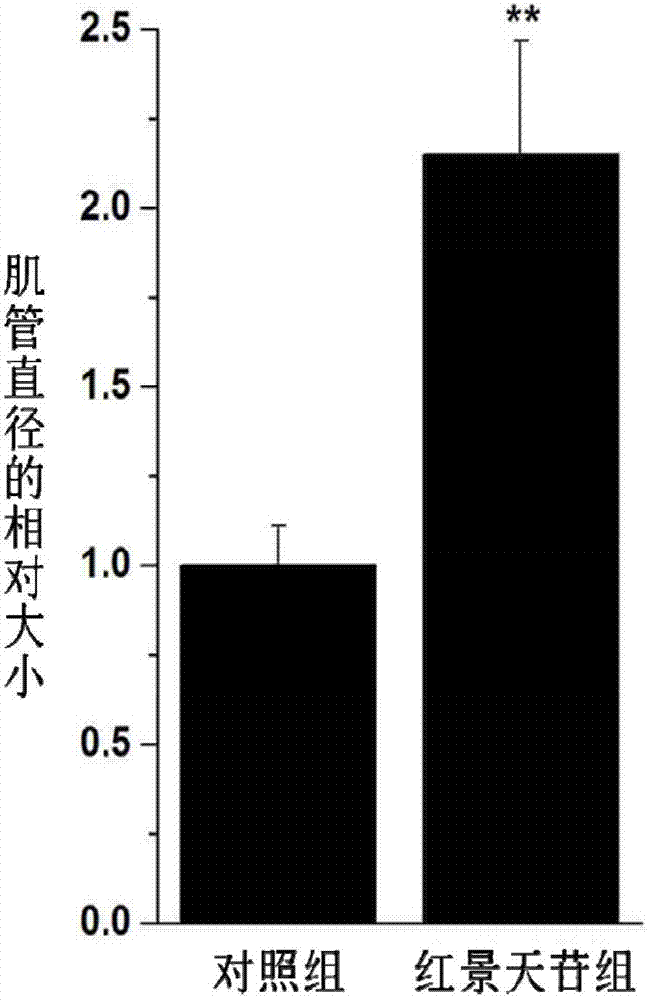
Application of salidroside in preventing and treating amyotrophy diseases
A technology of salidroside and muscle atrophy, which is applied in muscular system diseases, neuromuscular system diseases, medical preparations containing active ingredients, etc., and can solve the problems affecting daily work and life, fatigue, and disuse muscle atrophy ( Long-term bed rest, traumatic braking, and weightless muscle atrophy during spaceflight, etc., to reduce the expression of muscle atrophy-specific factors and increase the content of slow muscle fiber protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0038] The sources of experimental animals and materials used in the following experiments are as follows:
[0039] Experimental animals: male C57 mice were purchased from Beijing Weitong Lihua Animal Center, production license number: SCXK (Beijing) 2012-0001; experimental animal quality certificate number: 0261086.
[0040] The C2C12 cell line was purchased from the Chinese Academy of Medical Sciences.
[0041] Myotubes are the multinucleated syncytia formed by monocyte C2C12 in vitro myogenic differentiation prepared in this experiment.
[0042] Test drug: salidroside, purchased from Chengdu Pusi Biotechnology Co., Ltd., HPLC: 99%.
[0043] Additional material sources:
[0044] Tissue embedding agent was purchased from Thermo company;
[0045] Rabbit-derived laminin antibody was purchased from Abcam;
[0046] Goat anti-rabbit FITC fluorescently labeled secondary antibody was purchased from Invitrogen;
[0047] Goat anti-mouse FITC fluorescently labeled secondary antibo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com