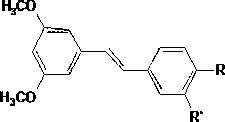
Water-soluble derivative of diphenylethene compounds, preparation method and usage thereof
A stilbene-based, water-soluble technology, applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, medical preparations containing active ingredients, etc., can solve problems such as increased cost and weak water solubility , to achieve the effect of low cost, high efficiency, simple and reliable preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
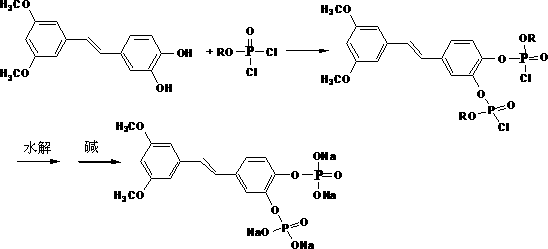
[0038] Preparation of Trans-4- [2- (3,5-dimethyl oxygenyl) ethylene group] -1, 2-benzhenyl phosphate di sodium salt
[0039] (O-Shu Ding) -TRANS-4- [2- (3, 5-dimer oxygenyl) ethylene group] -1, 2-benzhenol phosphate 10 g, methanol 100 ml add 500 ml to 500 mLIn the reaction bottle, the dry hydrogen chloride gas is applied in an appropriate amount, the temperature is increased to 40-50 degrees for 24 hours, filtering, decompression to remove solvents, the product is consolidated with acetone, the phosphate of the compound, filtering, drying, and 2 m NaOH of 2 m NaOHIn the methanol, the salt, filtering, adding acetone to analyze white solids, and 6.1 grams of dry products.The compound of the income compound performed a nuclear magnetic resonance analysis, and the results were shown in Table 1.
[0040] Table 1 TRANS-4- [2- (3,5-dimer oxygenyl) ethylene groups] -1, 2-benzhenyl phosphate di-sodium salt duty magnetic resonance data
[0041] (( 1 H nmr 500 mHz, 13 C NMR 500 MHz in CDCL ...
Embodiment 2
[0043] Example 2 Trans-4- [2- (3, 5-dimer oxygenyl) ethylene groups] -1, 2-phenyl alcohol phosphate sodium saline test
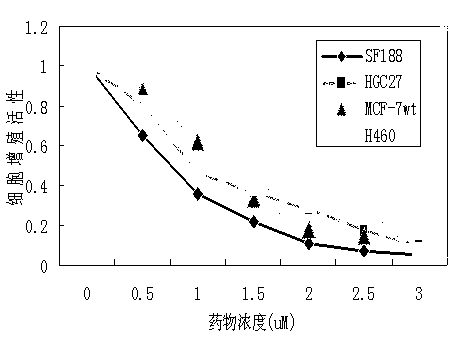
[0044] Take the correct number of cells 3 × 10 per hole 4 After inoculation on the 96 -hole board, after 12 hours, abandon the Qing Dynasty, and click the group to administer: the normal control group and the addition of the medicine group (concentration 0-100 μm), each group is set 6 to cultivate 24h, abandon the Qingqing, discard the Qing Dynasty, discard the clearing, and abandon the Qing Dynasty.Add 50 μl of medium with MTT to cultivate 4H (0.5 mg / ml), add 100 μL DMSO, oscillate 1h, and test the OD value at 570 nm on the enzyme marker.
[0045]The results showed that after adding medicine, cell activity decreased significantly, and cell activity decreased with the increase of drug concentration.For SF188 (cerebral gel tumor cells), HGC-27 (gastric cancer cells), MCF-7WT (breast cancer cells), H460 (large cell lung cancer cells) (cell strains are purchased fr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com