Solid-phase synthesis method of Cilengitide
A cilengitide and solid-phase synthesis technology, which is applied in the field of solid-phase cyclization technology to synthesize cyclic peptides, can solve problems such as unfavorable industrial production, reduced cyclization yield, troublesome purification, etc., and achieves low cost, simple post-processing, The effect of short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
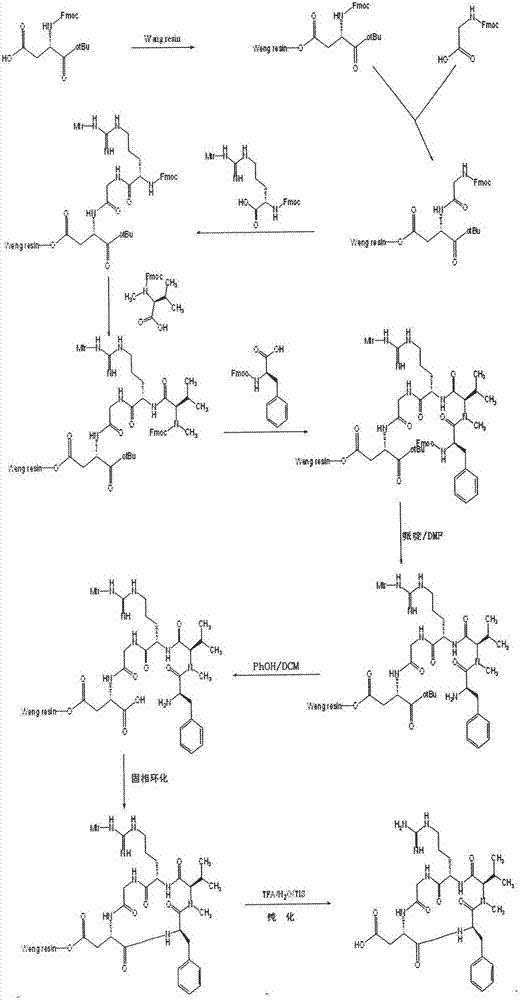
[0027] Preparation of Fmoc-L-Asp(OtBu)-Wang Resin
[0028] Weigh 10g of Wang Resin with a substitution degree of 0.5mmol / g into the reactor, add an appropriate amount of DCM, swell for 30min, and remove the DCM; dissolve 6.17g of Fmoc-L-Asp-OtBu, 2.40ml of DIC, and 2.1g of HOBT In 30ml of DMF, activate at 0-5°C for 15min, add the activation solution into the reactor containing Wang Resin, react for 10min, then add 0.18g of DMAP, react at 0-30°C for 1-5h. After the reaction, add 1ml of acetic anhydride and 0.5ml of pyridine to block the unreacted hydroxyl reagent of Wang Resin, and after 1 hour of blocking reaction, wash with 80ml of DMF, DCM, CH 3 Wash with OH and DMF 2, 1, 1, 2 times, 1 min each time. After testing, Fmoc-L-Asp(OtBu)-Wang Resin with a substitution degree of 0.47mmol / g was obtained.
Embodiment 2
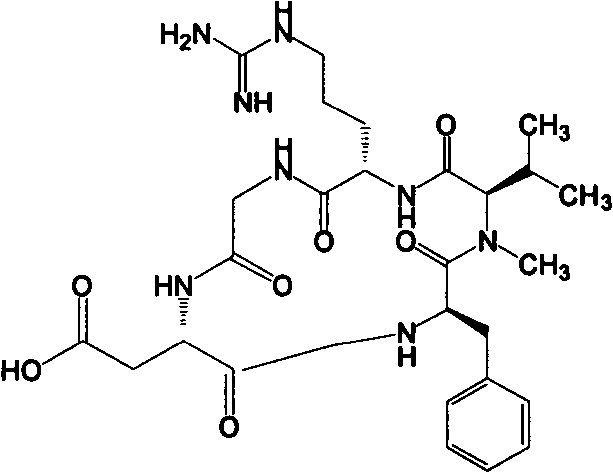
[0030] Cilengitide precursor:
[0031] Preparation of A-Wang Resin (Fmoc-D-Phe-N-Me-L-Val-L-Arg(Mtr)-Gly-L-Asp(OtBu)-Wang Resin)
[0032] Add Fmoc-L-Asp(OtBu)-Wang Resin to the reactor, swell with DMF for 30min, remove the solvent, add 80ml 25% piperidine-DMF to react for 5min, wash with 80ml DMF once (3min), add 80ml 25% piperidine-DMF reaction for 15min; wash with 80ml DMF, DCM, CH 3 Wash 2, 1, 1, 2 times with OH and DMF, 1 min each time; dissolve 4.45g Fmoc-Gly-OH, 5.68g HBTU, 2.03g HOBt in 30ml of DMF, add 2.45ml of DIEA after dissolution, 0 Activate at ~5°C for 15 minutes, add the activation solution into the above reactor, react at 0-30°C for 1-3 hours, and detect the end point of the reaction with the ninhydrin method. Using the above method to sequentially couple Fmoc-L-Arg(Mtr)-OH, Fmoc-N-Me-L-Val, Fmoc-D-Phe-OH, finally get Fmoc-D-Phe-N-Me-L-Val -L-Arg(Mtr)-Gly-L-Asp(OtBu)-Wang Resin.
Embodiment 3
[0034] Precursor peptide of cilengitide: preparation of B-Wang Resin (D-Phe-N-Me-L-Val-L-Arg(Mtr)-Gly-L-Asp-Wang Resin)
[0035] Be the Fmoc deprotecting agent of Fmoc-D-Phe-N-Me-L-Val-L-Arg(Mtr)-Gly-L-Asp(OtBu)-Wang Resin with volume ratio of 25% piperidine-DMF, Add 25% piperidine-DMF 80ml for the first time, react for 5min, wash with 80ml DMF once (3min), add 25% piperidine-DMF 80ml for the second time, react for 15min, wash with 80ml DMF, DCM in turn 、CH 3 Wash 2, 1, 1, 2 times with OH and DMF, each time for 1 min. After washing, D-Phe-N-Me-L-Val-L-Arg(Mtr)-Gly-L-Asp(OtBu)-Wang Resin.
[0036] Add 80% PhOH-DCM solution with a volume ratio of 100ml and a catalytic amount of TFA to remove OtBu, react for 8h; wash with 80ml of DMF, DCM, CH 3 Wash 2, 1, 1, 2 times with OH and DMF, 1 min each time, to obtain D-Phe-N-Me-L-Val-L-Arg(Mtr)-Gly-L-Asp-Wang Resin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com