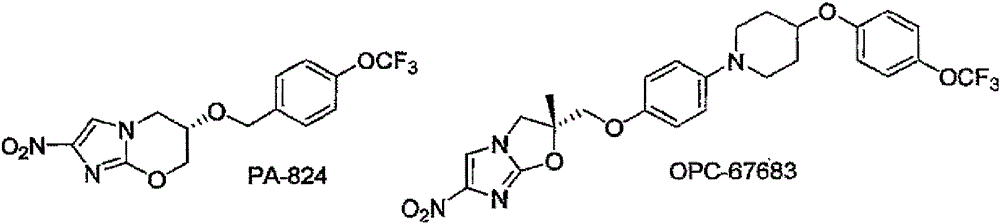
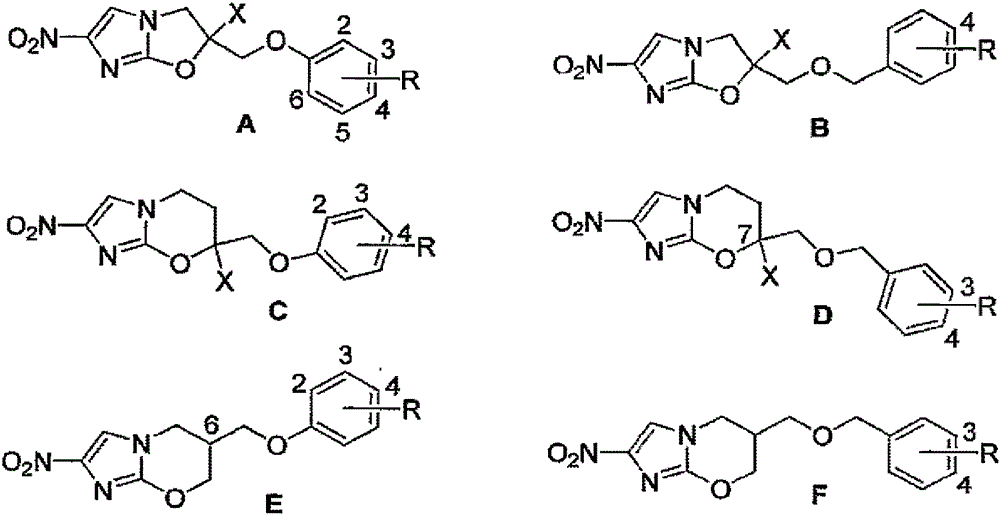
Nitroimidazoxazines and analogs of nitroimidazoxazoles and uses thereof
A technology of imidazo and nitro, which is applied in the fields of nitroimidazooxazine and nitroimidazooxazole analogues and their uses, and can solve the problems of increasing drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 1
[0166] Example 1. General Synthetic Scheme
[0167] It can be achieved through the listed in Scheme 1-15, Figure 3-17 Compounds are prepared according to the general methods shown or any other suitable method. In the descriptions of Scenarios 1-15 below, refer to Table 1 below and the attached figure 2 and 18 Representative compounds shown in -21.
[0168] Table 1: Representative Compounds
[0169]
[0170]
[0171]
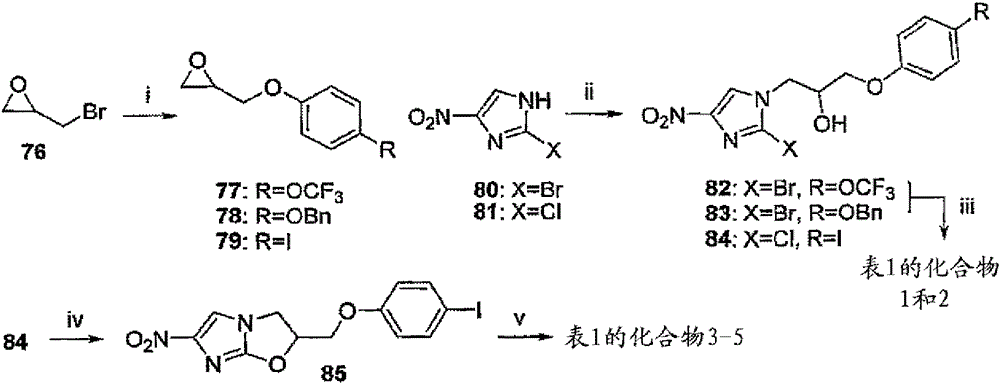
[0172] In Scenario 1, such as image 3 As shown, the reagents and conditions are (i) RPhOH, K 2 CO 3 , acetone, reflux, 36~52 hours; (ii) 77, 78 or 79, DIPEA, 105 ℃, 6.5~12 hours; (iii) NaH, DMF, 0 ℃, 45 minutes; (iv) NaH, DMF, 0 ℃, 80 minutes, then 17℃, 60 minutes; (v) ArB(OH) 2 , 2MNa 2 CO 3 , toluene, EtOH, DMF, N 2 Lower Pd(dppf)Cl 2 , 88-90°C, 50-90 minutes. 2-bromo-4(5)-nitroimidazole (80) or 2-chloro-4(5)-nitroimidazole (81) and epoxy 77~79 [by using 2-(bromomethyl) epoxy Prepared by alkylation of the appropriate 4-substituted phen...
Embodiment 2
[0187] Embodiment 2: preparation method
[0188] A. Synthesis of 6-nitro-2-{[4-(trifluoromethoxy)phenoxy]methyl}-2,3-dihydroimidazo[2,1-b][ 1,3] Oxazole (Compound 1 of Table 1).
[0189]
[0190] Stir 4-trifluoromethoxyphenol (0.152 mL, 1.17 mmol), K 2 CO 3 (260 mg, 1.17 mmol) and 2-(bromomethyl)oxirane (76) (0.30 mL, 3.51 mmol) in anhydrous acetone (3 mL) for 36 hours. The resulting mixture was filtered with CH 2 Cl 2 After washing, the filtrate is evaporated to dryness and the residue is chromatographed on silica gel. With 0~15% CH 2 Cl 2 / pentane elution to obtain the first eluate, and then use 20-25% CH 2 Cl 2 2-{[4-(trifluoromethoxy)phenoxy]methyl}oxirane (77) as an oil (similar to the preparation of Cao et al., WO2008112483A2 using epichlorohydrin ) (260mg, 95%);
[0191] 1 HNMR (CDCl 3 )δ7.14 (brdd, J=9.0, 0.6Hz, 2H), 6.91 (dt, J=9.1, 3.0Hz, 2H), 4.23 (dd, J=11.1, 3.1Hz, 1H), 3.94 (dd, J =11.1, 5.7Hz, 1H), 3.34(m, 1H), 2.91(dd, J=4.8, 4.2Hz, 1H), 2.75(d...
Embodiment 3
[0616] Example 3. Biological Activity and Stability
[0617] The biological activity of the compounds of the present invention was evaluated as follows. The results are shown in Table 2 below.
[0618] (a) Minimum inhibitory concentration (MIC). Compound activity against M. tuberculosis replication was assessed using Alamar blue reagent (added on day 7) to measure growth (MABA) in an 8-day microplate-based assay (Collins et al., 1997; Falzari et al., 2005) . The lowest compound concentration that achieved >90% inhibition was considered the MIC. Compounds were screened for activity against bacteria in a non-replicating state (mimicking clinical persistence) using an 11-day high-throughput, luminescence-based low-oxygen-recovery assay (LORA), in which tuberculosis Mycobacteria, which contain a plasmid with an acetamidase promoter driving a bacterial luciferase gene, were first adapted to hypoxic conditions (Cho et al., 2007).
[0619] (b) Maximum cytotoxicity assay . Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com