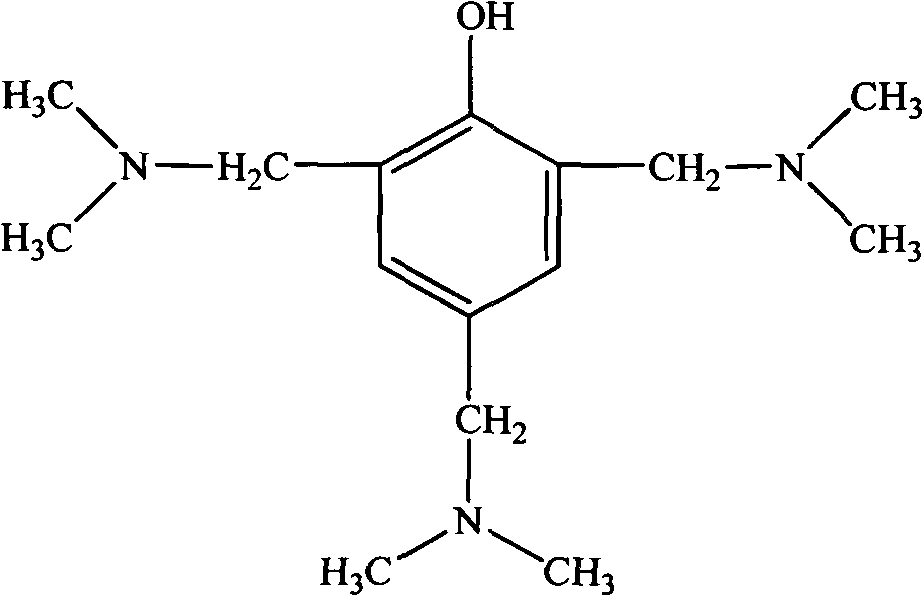
Method for synthesizing 2, 4, 6-tri(dimethylamino methyl) phenol
A technology of dimethylaminomethyl and phenol is applied in the field of chemistry to achieve the effects of increasing production cost, reducing energy consumption and high transportation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
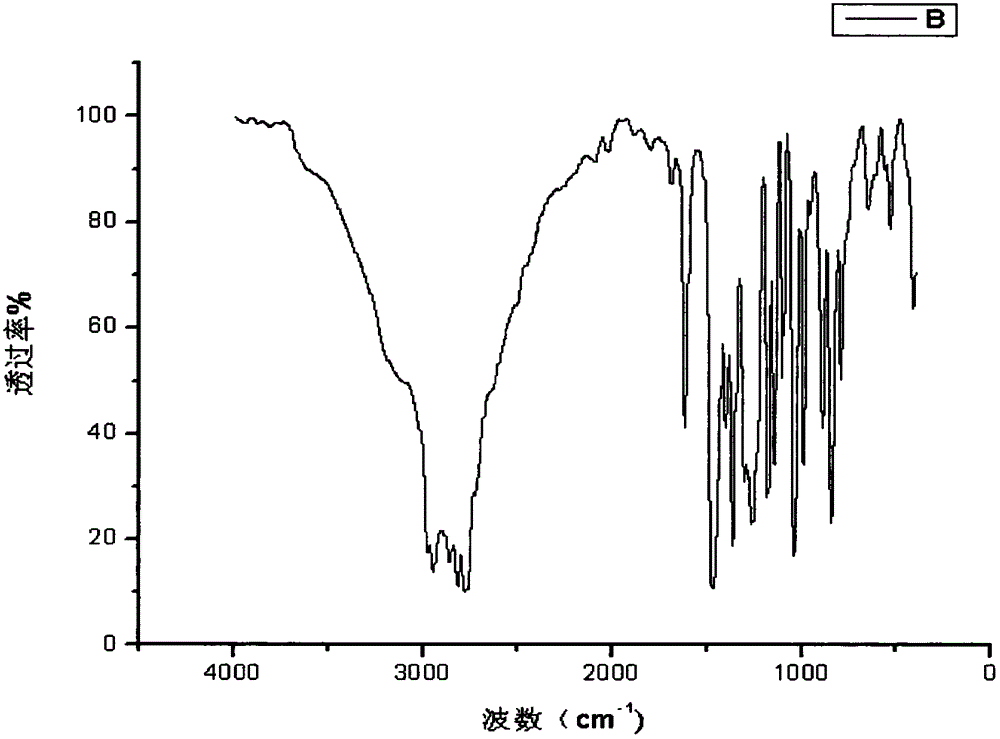
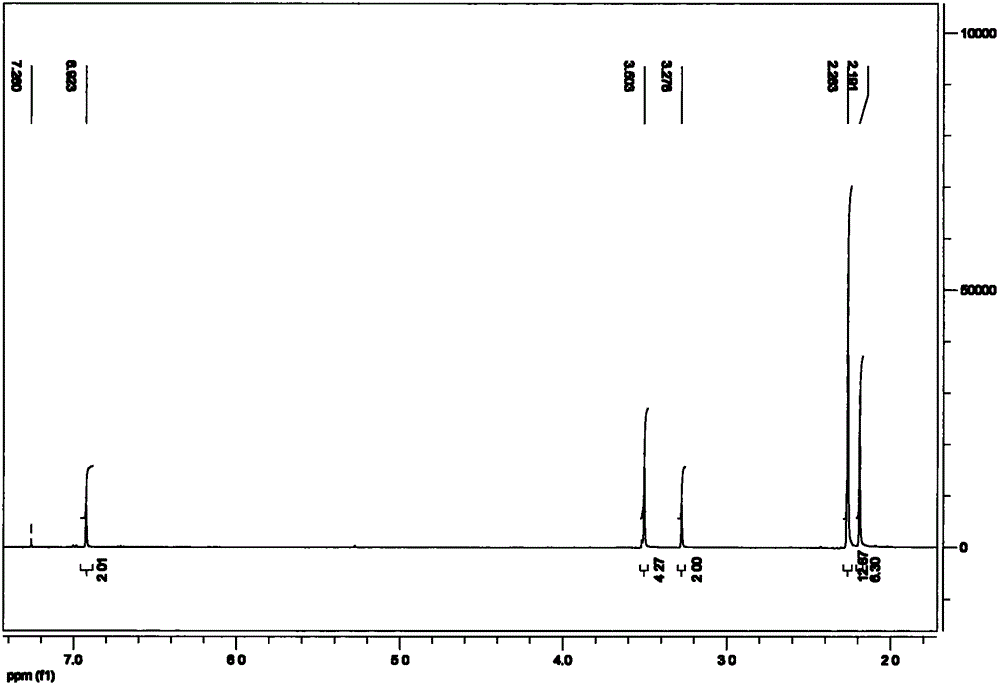
[0030] In a 500ml four-neck flask equipped with a stirring, reflux device, and a thermometer, add 28.2g of phenol and 135g of dimethylamine aqueous solution, and stir at room temperature for 15-30min to make it evenly mixed; then at a temperature of 20-50°C, Add 28.4g of paraformaldehyde several times within 1 hour; react the mixture at 70-80°C for 2-3 hours; then carry out oil-water separation and vacuum distillation to remove water from the product, and collect the product. The obtained product is a light yellow transparent liquid with a viscosity of 150-250mpa·s and an amine value of 600-630mgKOH / g. Its infrared spectrum is as attached figure 1 As shown, the H NMR spectrum is shown in the attached figure 2 shown. The amount of waste water produced after the reaction was about 112g. And as an accelerator, the effect is obvious.
Embodiment 2
[0034] Add 355kg of phenol and 1544kg of dimethylamine aqueous solution into the reaction kettle, and mix them uniformly at room temperature; then add 358kg of paraformaldehyde powder into the reaction kettle in fractions within 1 hour at a temperature of 20-50°C , make the mixed solution react at 70-80°C for 2-3 hours; then carry out oil-water separation and vacuum distillation to remove the water in the product, and collect the product. The obtained product is a light yellow transparent liquid with a viscosity of 150-250mpa·s and an amine value of 600-630mgKOH / g. The amount of waste water produced after the reaction is about 1300kg.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Amine value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com