Argatroban injection for resisting thrombus and preparation method thereof
A technology of argatroban and injection, which is applied in the field of western medicine injection preparation, can solve the problems of unsuitability for industrial production and complex preparation process, etc., and achieve inhibition of arterial-venous bypass thrombosis in rats, increase curative effect, and reduce bleeding The effect of adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-4
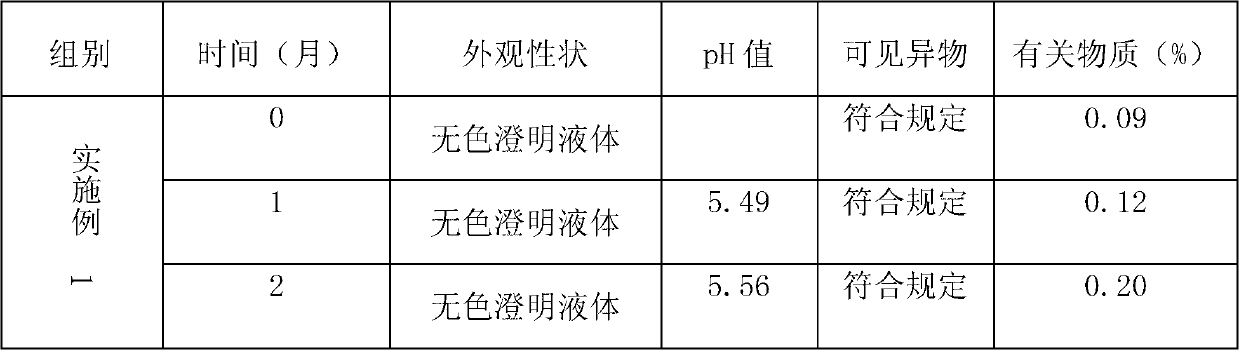
[0018] The prescription content of table 1 embodiment 1-4 argatroban injection
[0019] Component content
[0020] Preparation process: Add D-sorbitol and nitroglycerin to part of the water for injection, stir until dissolved, add 0.1% (W / V) activated carbon for needles, stir well, filter for decarbonization, add argatroban to the filtrate, and Heating while stirring, maintaining the temperature of the liquid medicine at 55-60°C, stirring until the argatroban is completely dissolved, adjusting the pH of the liquid medicine to 5.5-6.0 with 0.1mol / L hydrochloric acid solution or 0.1mol / L sodium hydroxide solution, Add the remaining amount of water for injection to constant volume, sterilize and filter with a 0.22 μm microporous membrane, fill, and sterilize with moist heat to obtain a clear solution.
Embodiment 5
[0021] Embodiment 5 Stability investigation test of argatroban injection of the present invention
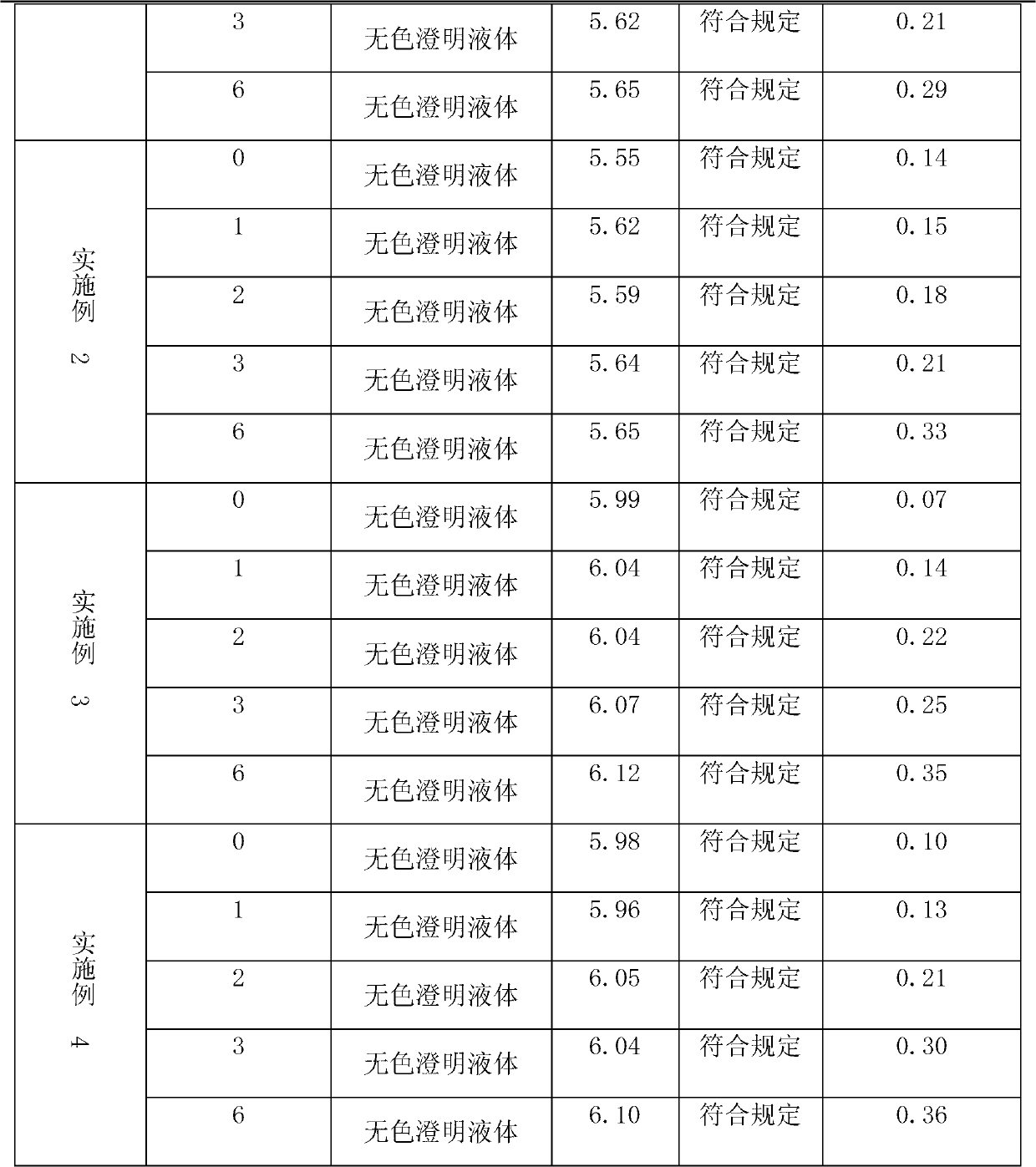
[0022] Take the argatroban injection prepared in Examples 1-4 of the present invention respectively, place them under the conditions of 40°C±2°C and RH75% to carry out the accelerated test, and take samples at the 1st, 2nd, 3rd, and 6th months respectively. The changes in appearance, pH value, visible foreign matter, and related substances were investigated respectively. The test results are shown in Table 2.
[0023] Table 2 each embodiment sample accelerated investigation result
[0024]
[0025]
[0026] As can be seen from the accelerated test results in Table 2, the argatroban injection prepared by the present invention is under accelerated conditions, and its appearance, pH value, visible foreign matter, and related substances all meet the requirements, and the content of related substances is low and increases over time. The change is not obvious, which shows that ...
Embodiment 6
[0027] Example 6 Inhibitory Effect of Argatroban Injection on Experimental Rat Carotid Artery-Venous Bypass Thrombosis
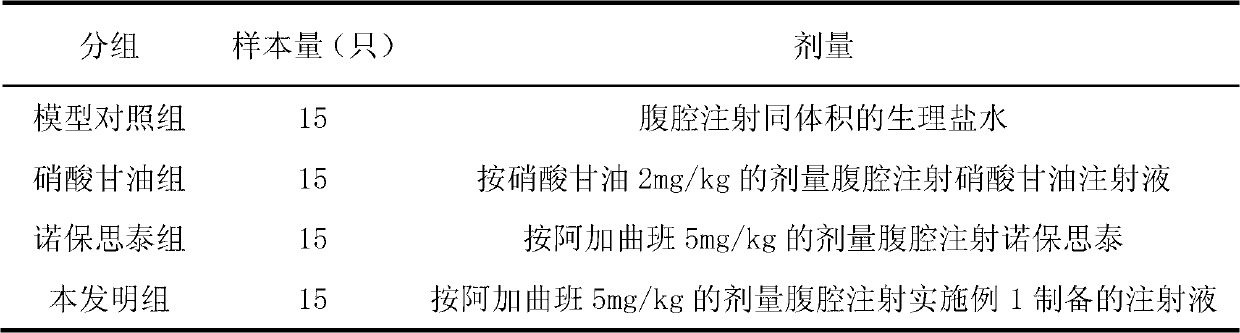
[0028] 60 rats, male, weighing 200-220 g, were randomly divided into 4 groups, 15 rats in each group, and raised for one week in advance. A model control group, a nitroglycerin group, a Noblast group and a group of the present invention were established. Each group was administered in the manner shown in Table 3:
[0029] Table 3 Grouping and administration of experimental animals
[0030]
[0031] Note: Nuobaositai is argatroban injection produced by Japan Mitsubishi Pharmaceutical Co., Ltd., specification 20mL: 10mg; nitroglycerin injection is produced by Sihuan Pharmaceutical Co., Ltd., specification 1ml: 5mg.
[0032] Rats were weighed, and 1 hour after administration, anesthetized by intraperitoneal injection of urethane, the left external jugular vein and the right common carotid artery were separated, and a cannula composed of three polyethylene ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com