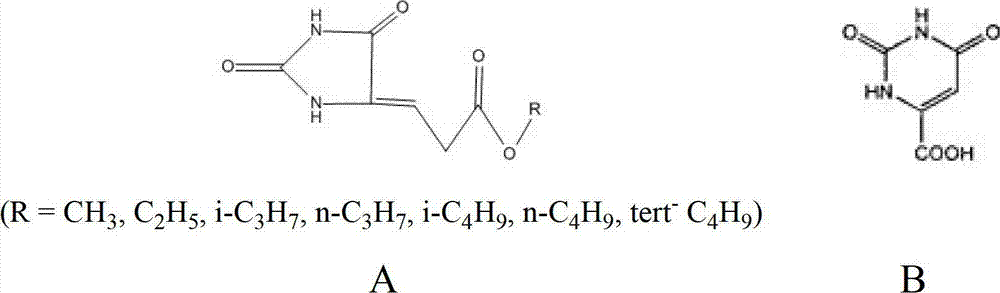
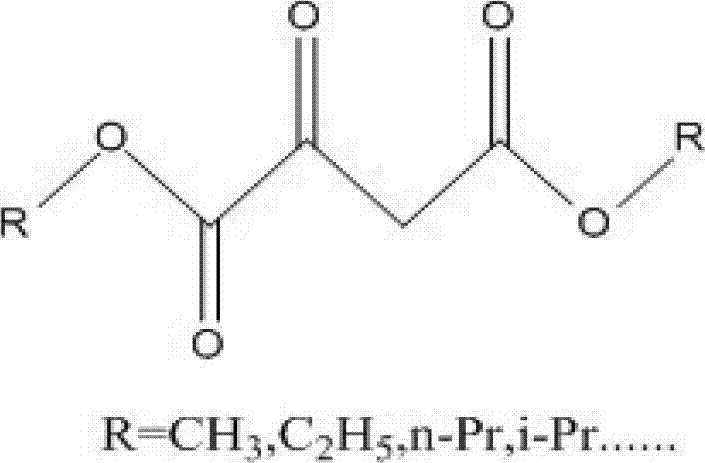
Synthetic method for orotic acid intermediate 5-alkoxy methylene hydantoin
A technology of alkoxymethylene hydantoin and synthesis method, which is applied in the field of synthesis of orotic acid intermediate 5-alkoxymethylene hydantoin, and can solve complex post-processing, pollution, and consumption of concentrated sulfuric acid Large volume and other problems, to achieve the effect of simple and economical treatment process, improved operating environment, and less three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Get 200g 2-keto-dimethyl succinate and heat to dissolve in 1L xylene, then mix 113g urea, 20gSO 4 2- / ZrO 2 , put into 2L xylene, start heating and stirring, 2-keto-dimethyl succinate (R=CH 3 ) xylene solution is slowly dropped into the mixture of urea, solid super acid and xylene, the temperature of the oil bath is controlled at 130°C, and the reaction is carried out for 8-24 hours. When the reaction is finished, cool to room temperature, filter, and continue to use the filtered solid superacid, concentrate the mother liquor to obtain 5-methoxymethylenehydantoin, and the reaction yield is 72%. Washed twice with ethanol, directly used for the reaction of orotic acid in the next step.
Embodiment 2
[0020] Get 50g of 2-keto-dimethyl succinate and dissolve it in 200ml of toluene with heating, then add 28.3g of urea, 5gSO 4 2- / Fe 2 o 3 -Al 2 o 3 , put into 500ml toluene, start heating and stirring, 2-keto-dimethyl succinate (R=CH 3 ) toluene solution slowly drop into the mixture of urea, solid superacid and toluene, the oil bath temperature is controlled at 130°C, and the reaction is 8-24 hours. When the reaction is finished, cool to room temperature, filter, and continue to use the filtered solid superacid, concentrate the mother liquor to obtain 5-methoxymethylenehydantoin, and the reaction yield is 68%. Washed twice with ethanol, directly used for the reaction of orotic acid in the next step.
Embodiment 3
[0022] Get 84.9g urea, 15g solid super acid SO 4 2- / ZrO 2 -Al 2 o 3 , put into 1L xylene, and drop into 2-keto-diethyl succinate (R=C 2 h 5 ) 165g, start heating and stirring, in the mixture of xylene, the oil bath temperature is controlled at 130°C, react for 8-24 hours, when the reaction is over, cool to room temperature, filter, and continue to use the filtered solid superacid, concentrate the mother liquor , That is, 5-ethoxymethylene hydantoin was obtained, and the reaction yield was 74%. Washed twice with ethanol, directly used for the reaction of orotic acid in the next step.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com