Synthesis method of taxane drug 7, 10-methoxy-docetaxel
A synthetic method and taxane technology, applied in organic chemistry, bulk chemical production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
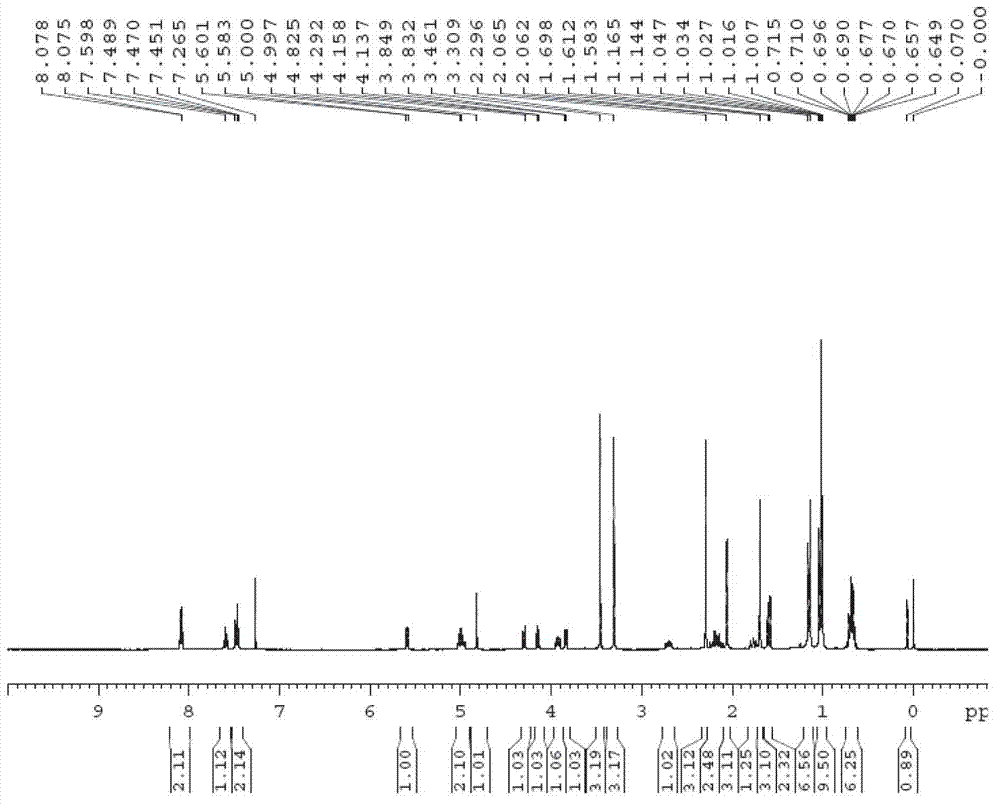
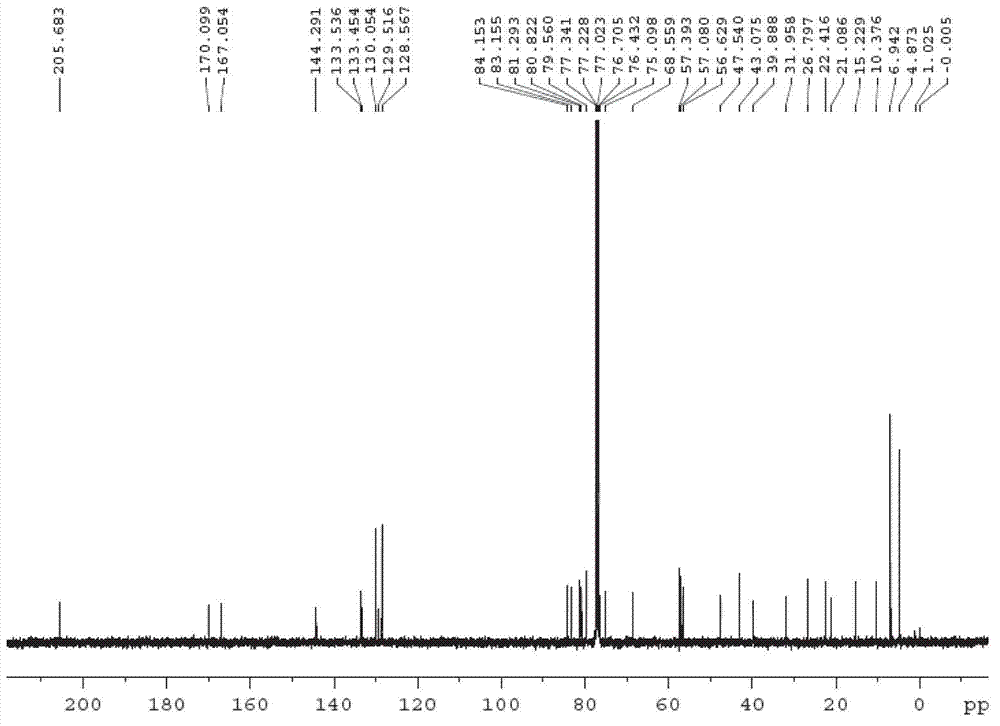
[0052] Embodiment 1 (R is a triethylsilyl protecting group, TES-)
[0053] Step 1: 7,10-Hydroxy-13-triethylsilyloxy-10-desacetylbaccatin III (compound 2 ) preparation
[0054] 7,10-trichloroethoxycarbonyl-10-desacetylbaccatin III (compound 1 ) (50 g, 55.9 mmol) was fully dissolved in DMF (500 mL), then 2-methylimidazole (6.9 g, 83.8 mmol) was added, and triethylchlorosilane (11.3 mL, 67.0 mmol) was added dropwise and the reaction was stirred at room temperature After 12 hours, 2000 mL of water was added to the reaction solution, extracted with ethyl acetate (300 mL×3), the organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, and filtered with suction. Add zinc powder (4.4 g, 67.0 mmol), methanol (18 mL), acetic acid (3.8 mL, 67.0 mmol) to the filtrate, stir at room temperature and react for 12 hours, filter with suction, concentrate, add 500 mL of acetonitrile to the reaction solution, and react A large amount of solids precipitated out in t...
Embodiment 2
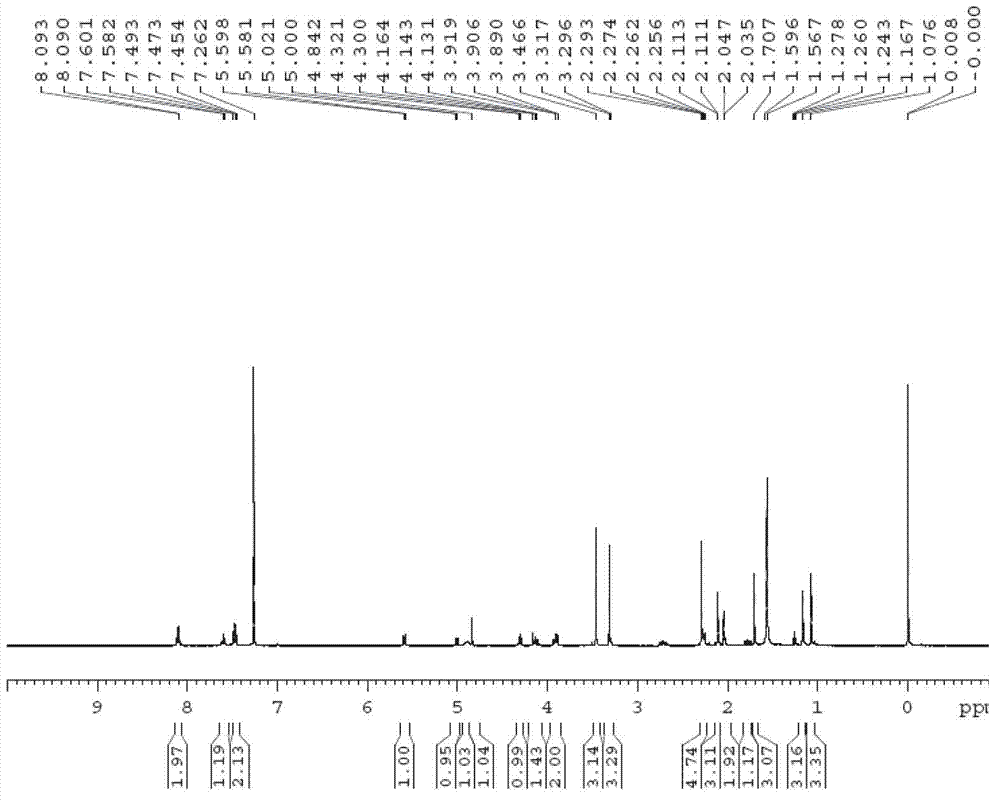
[0065] Example 2 (R is a tert-butyldimethylsilyl protecting group, TBS-)
[0066] Step 1: 7,10-hydroxy-13-tert-butyldimethylsilyloxy-10-deacetylbaccatin III (compound 2 ) preparation
[0067] 7,10-trichloroethoxycarbonyl-10-desacetylbaccatin III (compound 1 ) (50 g, 55.9 mmol) was fully dissolved in DMF (500 mL), 2-methylimidazole (6.9 g, 83.8 mmol) was added, and tert-butyldimethylsilyl chloride (11.3 mL, 67.0 mmol) was added dropwise After stirring and reacting at room temperature for 12 hours, 2000 mL of water was added to the reaction liquid, extracted with ethyl acetate (300 mL×3), the organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, and filtered with suction. Add zinc powder (4.4 g, 67.0 mmol), methanol (18 mL), acetic acid (3.8 mL, 67.0 mmol) to the filtrate, stir at room temperature and react for 12 hours, filter with suction, concentrate, add 500 mL of acetonitrile to the reaction solution, and react A large amount of solids pre...
Embodiment 3
[0078] Embodiment 3 (R is triethylsilyl protecting group, TES-)
[0079] Step 1: 7,10-Hydroxy-13-triethylsilyloxy-10-desacetylbaccatin III (compound 2 ) preparation
[0080] 7,10-trichloroethoxycarbonyl-10-desacetylbaccatin III (compound 1 ) (50 g, 55.9 mmol) was fully dissolved in DMF (500 mL), then 2-methylimidazole (4.6 g, 55.9 mmol) was added, and triethylchlorosilane (9.4 mL, 55.9 mmol) was added dropwise and the reaction was stirred at room temperature After 12 hours, 2000 mL of water was added to the reaction solution, extracted with ethyl acetate (300 mL×3), the organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, and filtered with suction. Add zinc powder (3.7 g, 55.9 mmol), methanol (18 mL), acetic acid (3.2 mL, 55.9 mmol) to the filtrate, stir and react at 20°C for 12 hours, filter with suction, concentrate, add 500 mL of acetonitrile to the reaction liquid, A large amount of solids were precipitated in the reaction solution, suct...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com