Catalyst for methylbenzene directional chlorination reaction and preparation method thereof
A chlorination reaction and catalyst technology, applied in the field of catalyst and its preparation, can solve the problems of low activity of L-type zeolite, easy collapse of pore structure, large amount of catalyst, etc., achieve high activity and p-chlorotoluene selectivity, and simple preparation method , the effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] According to K 2 O:Al 2 o 3 : SiO 2 :H 2 O=9.3:1:20:412 molar ratio of raw materials, weigh 54g of potassium hydroxide solid and 7.6g of aluminum hydroxide powder, put them in a 500ml round bottom flask, add 262g of deionized water, and reflux at 120°C for 0.5h After dissolving and cooling to room temperature, the aluminum alkali solution was transferred to a 500ml polytetrafluoroethylene beaker. Under vigorous stirring conditions, 145.6 g of 40 wt % silica sol was added dropwise. After stirring at room temperature for 1 h, a gel was obtained.
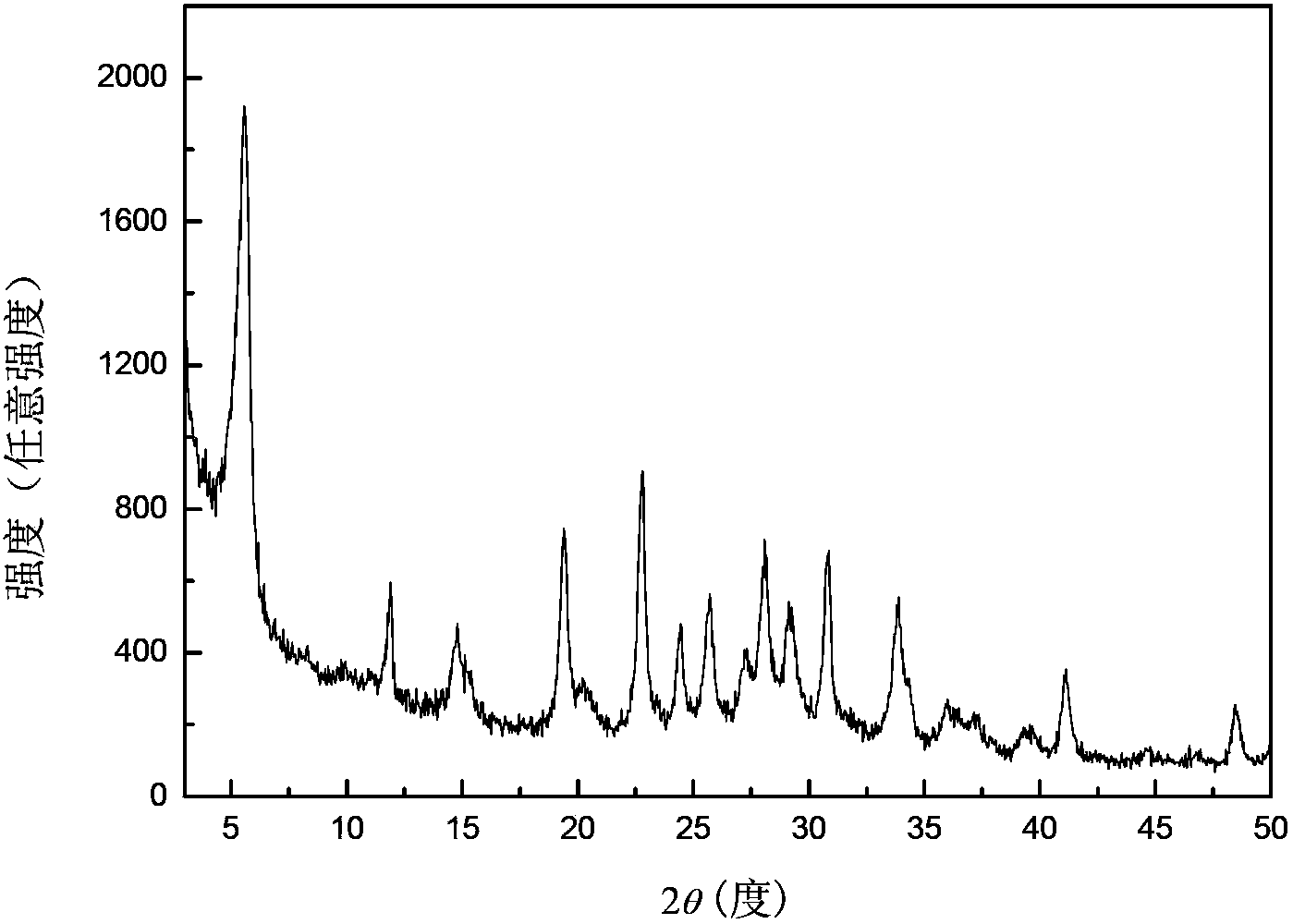
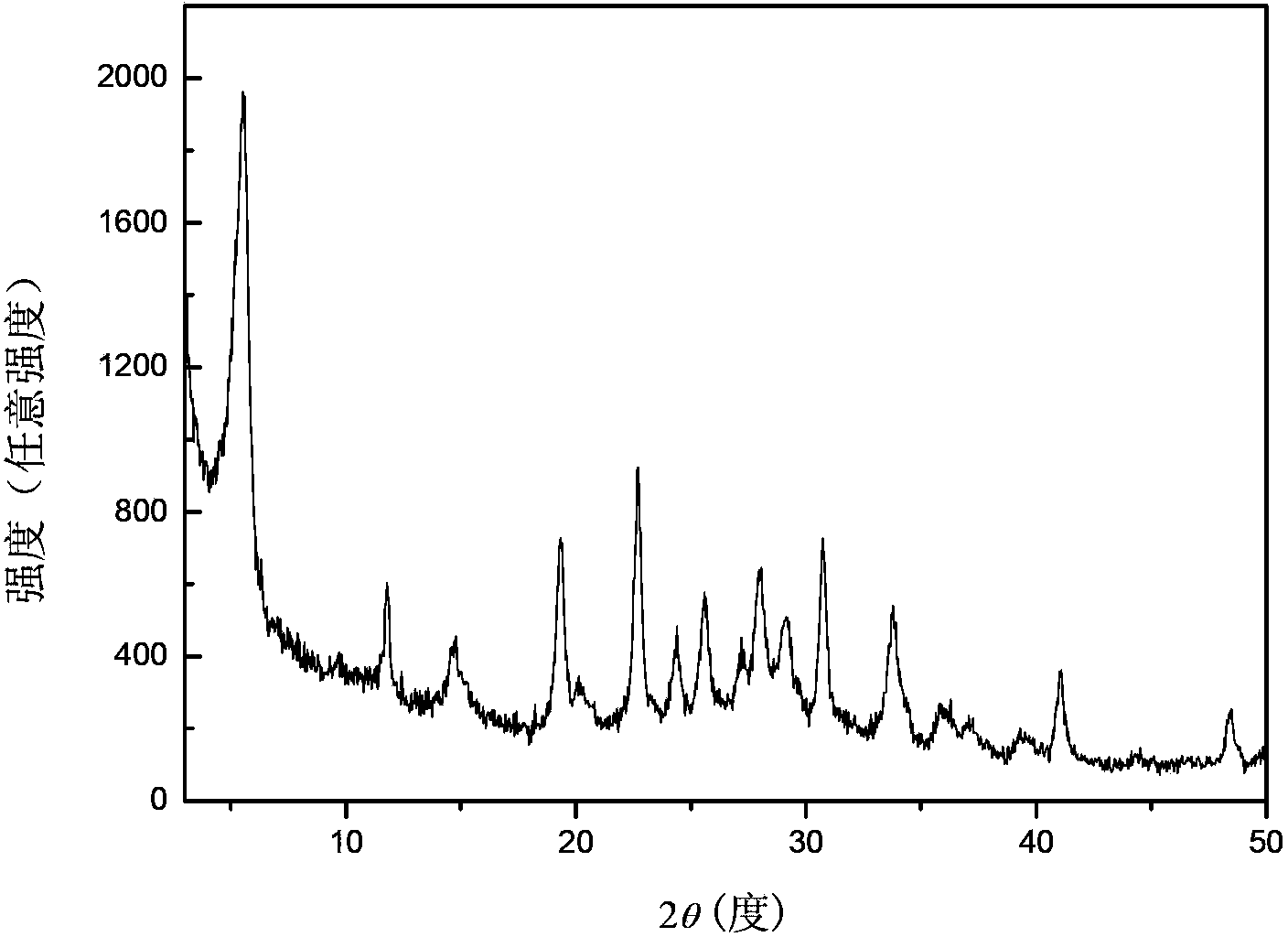
[0027] Transfer the above gel into a stainless steel autoclave lined with polytetrafluoroethylene, place it in an oven at 170°C for static crystallization for 6 hours, centrifuge, wash with hot water at 100°C until the supernatant is neutral, and store the sample at 100°C After drying for 12 hours, a white powder sample was obtained, and the sample was recorded as K 9 -L. The samples were characterized by X-ray diffracti...
Embodiment 2
[0033] According to K 2 O:Al 2 o 3 : SiO 2 :H 2 O=9.3:1:20:412 molar ratio of raw materials, weigh 54g of potassium hydroxide solid and 7.6g of aluminum hydroxide powder, put them in a 500ml round bottom flask, add 262g of deionized water, and reflux at 120°C for 0.5h After dissolving and cooling to room temperature, the aluminum alkali solution was transferred to a 500ml polytetrafluoroethylene beaker. Under vigorous stirring conditions, 145.6 g of 40 wt % silica sol was added dropwise. After stirring at room temperature for 1 h, a gel was obtained.
[0034] Transfer the above gel into a stainless steel autoclave lined with polytetrafluoroethylene, place it in an oven at 170°C for static crystallization for 6 hours, centrifuge, wash with hot water at 100°C until the supernatant is neutral, and store the sample at 100°C After drying for 12 hours, a white powder sample was obtained, and the sample was recorded as K 9 -L. The samples were characterized by X-ray diffracti...
Embodiment 3
[0040] According to K 2 O:Al 2 o 3 : SiO 2 :H 2 O=9.3:1:20:412 molar ratio of raw materials, weigh 54g of potassium hydroxide solid and 7.6g of aluminum hydroxide powder, put them in a 500ml round bottom flask, add 262g of deionized water, and reflux at 120°C for 0.5h After dissolving and cooling to room temperature, the aluminum alkali solution was transferred to a 500ml polytetrafluoroethylene beaker. Under vigorous stirring conditions, 145.6 g of 40 wt % silica sol was added dropwise. After stirring at room temperature for 1 h, a gel was obtained.
[0041] Transfer the above gel into a stainless steel autoclave lined with polytetrafluoroethylene, place it in an oven at 170°C for static crystallization for 6 hours, centrifuge, wash with hot water at 100°C until the supernatant is neutral, and store the sample at 100°C After drying for 12 hours, a white powder sample was obtained, and the sample was recorded as K 9 -L. The samples were characterized by X-ray diffracti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com