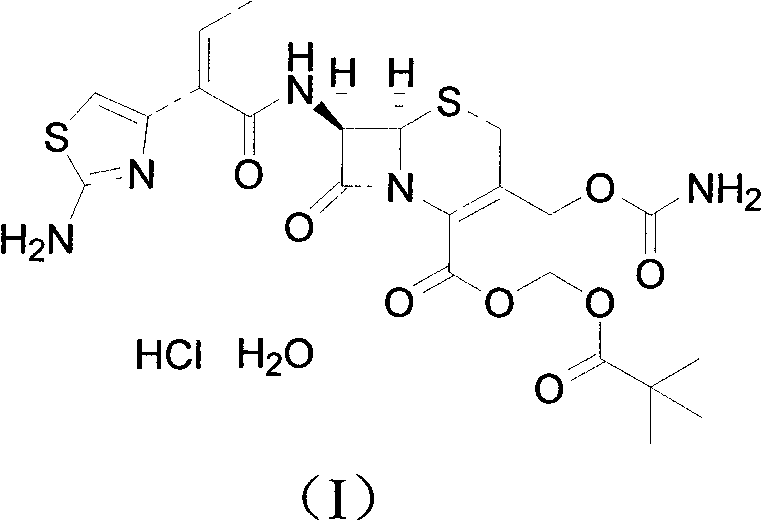
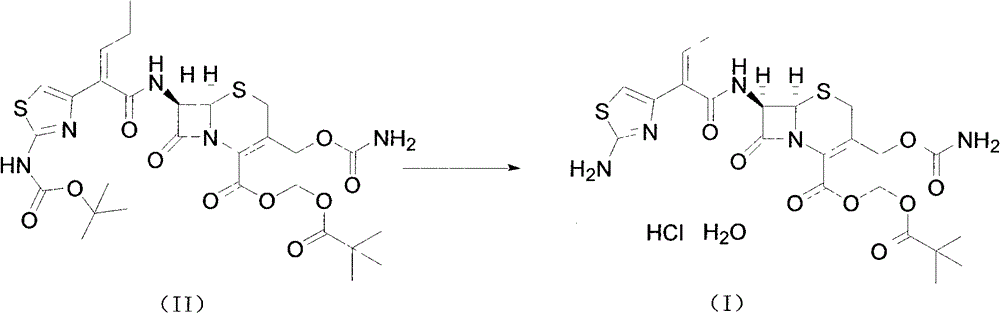
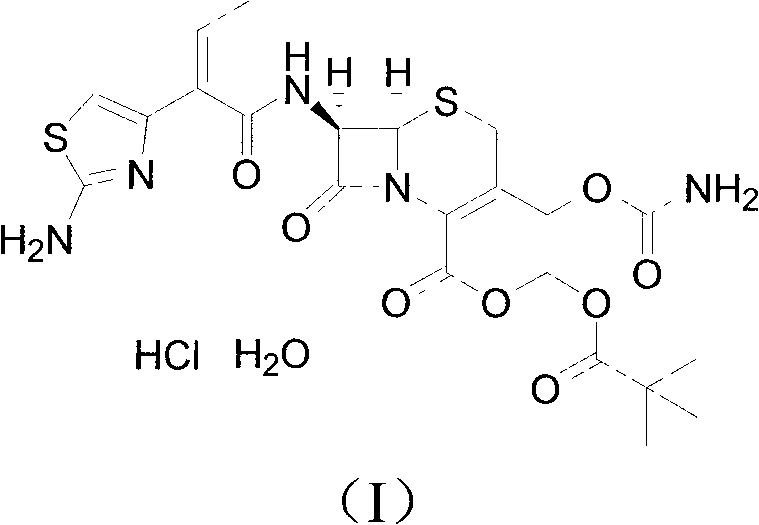
Method for preparing cefcapene pivoxil hydrochloride
A technology of cefcapine and hydrochloric acid, applied in the direction of organic chemistry, etc., can solve the problems of difficult processing, troubled industrial scale-up production, unstable materials, etc., and achieves the effects of high conversion rate, reduction of long-term storage, and rapid reaction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add ethyl acetate (1500mL) and aluminum trichloride (75g) to the reactor, stir to dissolve and cool down to -5~5°C, add compound of formula (II) (75g), and stir at -5~5°C React for 45 minutes. Water (1500 mL) was then quickly added to the reactor, stirred for 30 minutes, allowed to stand for 30 minutes, and the aqueous layer was drained. Wash with 10% sodium chloride solution (750mL), drain the water layer, control the temperature at 15-25°C, add 4mol / L hydrochloric acid (56.3mL) into the reactor, stir for 30 minutes, add acetonitrile (300mL), and stir for 3 hours . After centrifugation, the crude product was washed with water (500 mL×3), and dried for 16 hours to obtain the fine product of cefcapene hydrochloride monohydrate (60 g).
[0022] 1 H NMR (400MHz, d 6 -DMSO, 25°C): δ: 1.022(t, 3H), 1.157(s, 9H), 2.192-2.266(m, 2H), 3.445-3.501(d-d, 1H), 3.563-3.628(d-d, 1H), 4.597(d, 1H), 4.828(d, 1H), 5.231(d, 1H), 5.783-5.811(m, 2H), 5.891(d, 1H), 6.353(t, 1H), 6.477(...
Embodiment 2
[0024] Add ethyl acetate (1500mL) and aluminum trichloride (100g) into the reactor, stir to dissolve and cool down to -5~5°C, add the compound of formula (II) (75g), control the temperature at -5~5°C and stir React for 45 minutes. Water (1500 mL) was quickly added to the reactor, stirred for 30 minutes, left to stand for 30 minutes, and the water layer was drained. Wash with 10% sodium chloride solution (750mL), drain the water layer, control the temperature at 15-25°C, add 4mol / L hydrochloric acid (56.3mL) into the reactor, stir for 30 minutes, add acetonitrile (300mL), and stir for 3 hours . After centrifugation, the crude product was washed with water (500 mL×3), and dried for 16 hours to obtain the fine product of cefcapene hydrochloride monohydrate (55 g).
Embodiment 3
[0026] Add dichloromethane (1500mL) and aluminum trichloride (75g) into the reactor, stir to dissolve and cool down to -5~5°C, add the compound of formula (II) (75g), control the temperature and stir at -5~5°C React for 45 minutes. Water (1500 mL) was quickly added to the reactor, stirred for 30 minutes, left to stand for 30 minutes, and the water layer was drained. Wash with 10% sodium chloride solution (750mL), drain the water layer, control the temperature at 15-25°C, add 4mol / L hydrochloric acid (56.3mL) into the reactor, stir for 30 minutes, add acetonitrile (300mL), and stir for 3 hours . After centrifugation, the crude product was washed with water (500 mL×3), and dried for 16 hours to obtain the fine product of cefcapene hydrochloride monohydrate (55 g).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com