Application of pronucleus-derived ubiquitin-like molecules
A ubiquitin-like, prokaryotic technology, applied in the field of heterologous expression of recombinant proteins, can solve problems such as unknown sulfur transport molecules, and achieve the effects of increasing expression levels, increasing in vivo stability, and increasing the proportion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
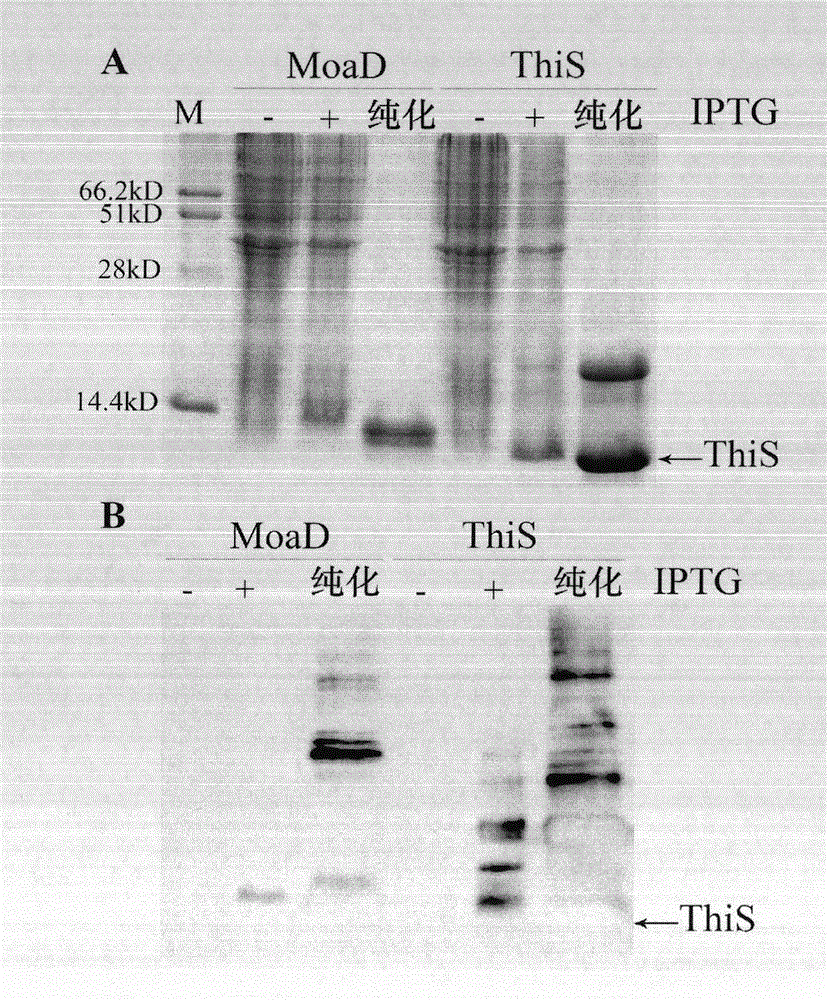
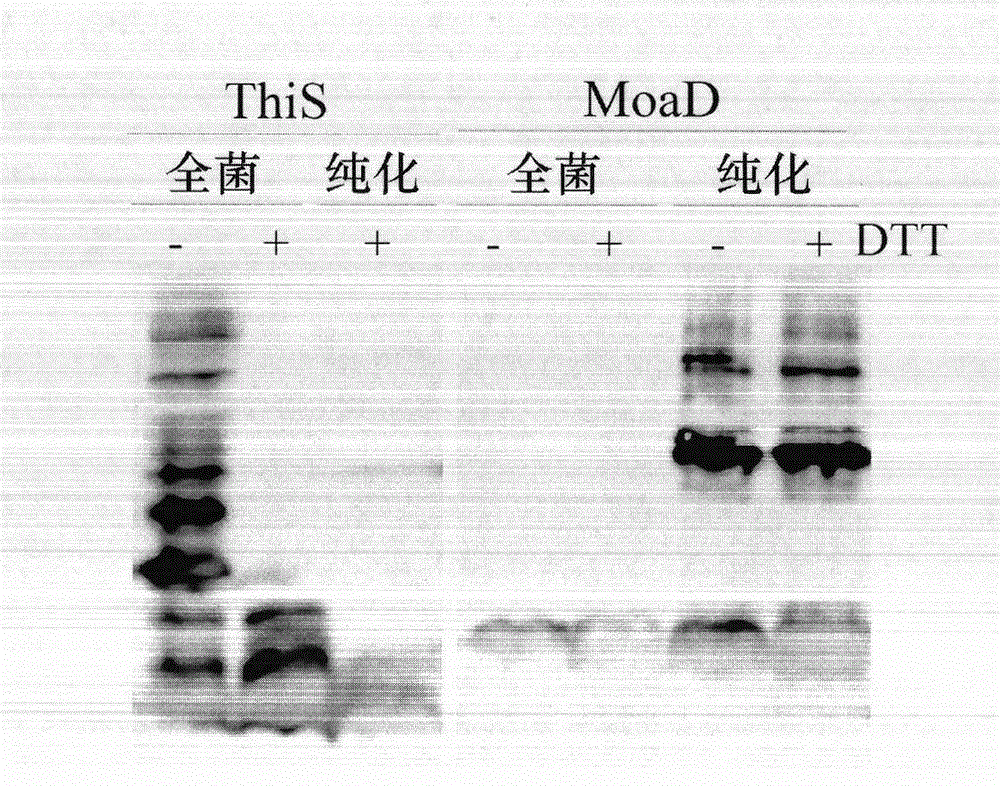
[0061] Example 1. The effect of fusion partner ThiS or MoaD on the expression of mouse ribonuclease inhibitor mRI.
[0062] In order to observe the fusion partner effect of ThiS and MoaD and compare with ubiquitin and Sumo, the fusion expression vector Ub-mRI / pVI and Sumo-mRI / pVI of ubiquitin and Sumo and mouse ribonuclease inhibitor mRI were constructed. And MoaD and ThiS and mRI fusion expression vector MoaD-mRI / pVI and ThiS-mRI / pVI. The fusion expression products all contain an amino-terminal polyhistidine tag. mRI is a protein that is prone to degradation in vivo (Ge Ying, et al. Chinese Journal of Bioengineering 2010, 30: 17-23), which is convenient for observing the effects of fusion partners. In TG1 and BL21(DE3)pLysS strains induced by IPTG, Ub-mRI and Sumo-mRI mainly contain complete fusion expression products, and many low-molecular-weight degradation bands can be seen; in BL21(DE3) lacking Lon protease In pLysS bacteria, product degradation is reduced ( image 3 A-B)...
Embodiment 2
[0065] Example 2. Fusion expression of fusion partner ThiS and human insulin A chain.
[0066] Human insulin A chain is a small polypeptide containing 21 amino acid residues, which needs to be expressed heterologously in the form of a fusion protein. A chain protein expression vector ThiS-A / pET28a and Ub-A / pET28a fused with ThiS and ubiquitin were constructed to promote the expression of human insulin A chain and to compare the fusion partner effects of ThiS and ubiquitin. The theoretical calculated molecular weights of ThiS-A and Ub-A fusion proteins are 13438 and 14561, respectively. After BL21(DE3)pLysS IPTG induction, both of them produced protein bands of expected size without obvious degradation products. However, the yield of ThiS fusion products is significantly higher than that of ubiquitin fusion products ( Image 6 ).
Embodiment 3
[0067] Example 3. Fusion expression of fusion partner ThiS and human insulin B chain.
[0068] The human insulin B chain is a small molecule polypeptide containing 30 amino acid residues, which also needs to be expressed heterologously in the form of a fusion protein. The B-chain protein expression vectors ThiS-B / pET28a and Ub-B / pET28a fused with ThiS and ubiquitin were constructed to promote the expression of human insulin B chain and to compare the fusion partner effects of ThiS and ubiquitin. The theoretical calculated molecular weights of ThiS-B and Ub-B fusion proteins are 14484 and 15607, respectively. After BL21(DE3)pLysS IPTG induction, both of them produced protein bands of expected size without obvious degradation products. However, the yield of ThiS fusion products is significantly higher than that of ubiquitin fusion products ( Figure 7 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com