Systems And Processes Of Operating Fuel Cell Systems
A fuel cell and feedstock technology, applied in fuel cells, regenerative fuel cells, fuel cell additives, etc., can solve problems such as low efficiency and insufficient heat
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
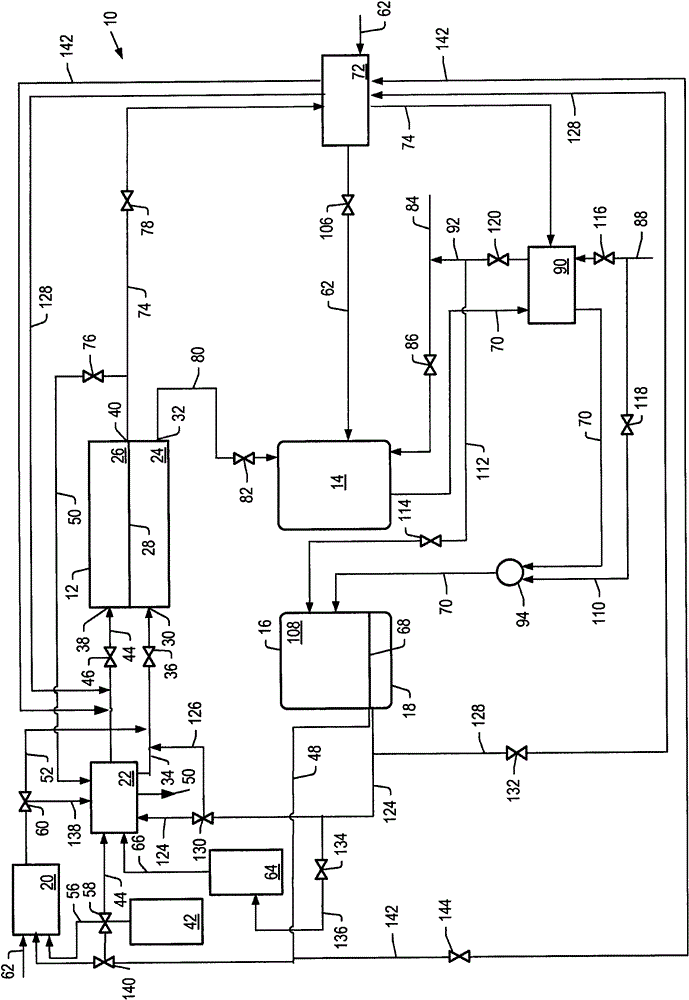
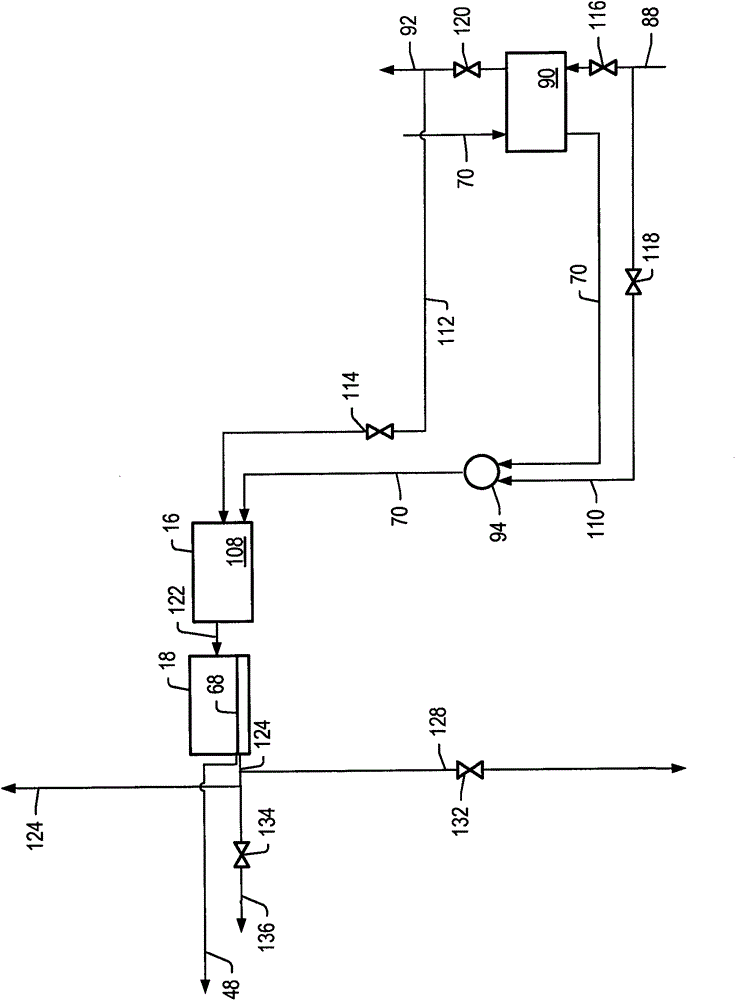
[0190] Example 1 uses the detailed process simulation described above to simulate cell voltage versus current density and power density development for the molten carbonate fuel cell system described herein, where the first reformer is heated by the anode exhaust and no other heating. E.g, figure 1 The system depicted. Heat for the second reformer is heated by exchange with the hot effluent from the catalytic partial oxidation reformer. The output temperature of the effluent from the catalytic partial oxidation reformer is increased by using the cathode exhaust to preheat the catalytic oxidation reformer air feed.
Embodiment 2
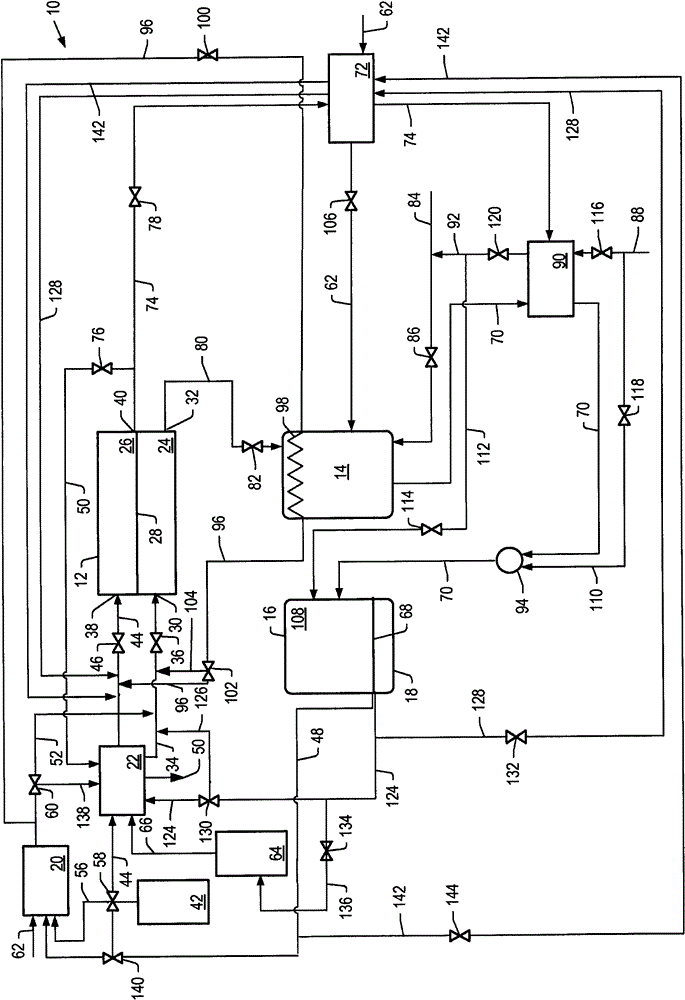
[0191] Example 2 uses the simulations described above to simulate cell voltage vs. current density and power density development for the molten carbonate fuel cell system described herein, with anode exhaust and heat from a catalytic partial oxidation reformer. The first reformer is heated. E.g, figure 2 The system depicted in .
[0192] For Examples 1 and 2, the molten carbonate fuel cell was operated at a pressure of 1 bar (about 0.1 MPa or about 1 atm) and a temperature of 650°C. The flow of feed to the cathode of the molten carbonate fuel cell is countercurrent to the flow of feed to the anode. Air was used as the oxygen source. The values for air were used to generate a molar ratio of carbon dioxide to molecular oxygen of 2 at various hydrogen utilization rates. The percent hydrogen utilization of the molten carbonate fuel cell simulated in Examples 1 and 2, the operating conditions of the first and second reformers, the ratio of steam to carbon, and the ratio of b...
Embodiment 3
[0201] Example 3 is directed to a molten carbonic acid fuel cell system (e.g., figure 1 system depicted in ), the simulations described above were used to determine the current density, cell voltage and power density of a molten carbonate fuel cell operating at 7 bar (about 0.7 MPa or about 7 atm). The molten carbonate fuel cells were operated at 20% or 30% hydrogen utilization at a pressure of 7 bar and a temperature of 650°C. The first reformer had a steam to carbon ratio of 2.5. The temperature of the first reformer is allowed to vary. The second reformer combined with the high temperature hydrogen separation unit has a temperature of 500° C. and a pressure of 15 bar. Air was used as the oxygen source. Values for air were used such that the ratio of carbon dioxide to molecular oxygen in the cathode feed was stoichiometric, thus minimizing cathode side concentration polarization. In all cases, the combined carbon conversion values for the systems using benzene as fee...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com