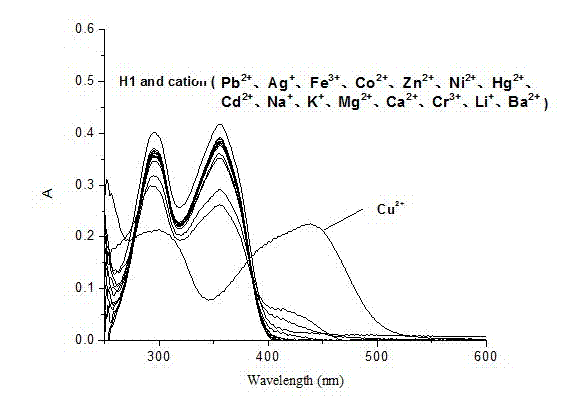
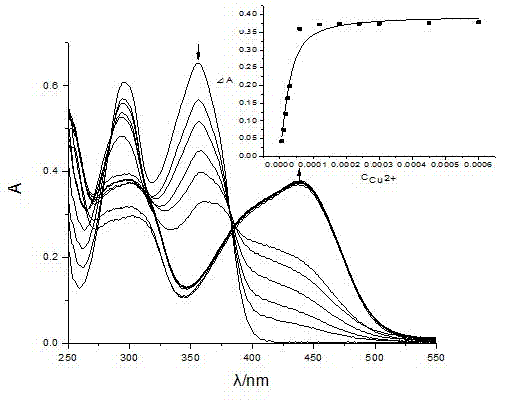
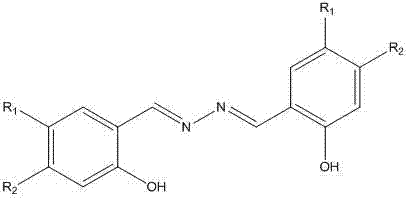
Salicylidenehydrazine receptor compound, preparation method and application thereof
A technology of salicylaldehyde hydrazide and compounds, which is applied in the preparation of hydrazone, the analysis of materials through chemical reactions, organic chemistry, etc., can solve the problems of long detection time, high cost, unfavorable copper element detection, etc., and achieve high yield High, simple process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of salicylaldehyde hydrazide receptor H1
[0037] Under the protection of nitrogen, add salicylaldehyde and hydrazine hydrate into a round bottom flask at a molar ratio of 2.5:1, add an appropriate amount of ethanol, reflux and stir for 4 hours, stop the reaction, filter, wash with ethanol, dry in vacuum, and recrystallize with ethanol to obtain salicylaldehyde hydrazide receptor.
[0038] Salicylaldehyde hydrazide receptor H1: khaki solid, m.p. 220.5-221.4℃; H1 yield: 83.3%; ESI-MS m / e 241.31, 1 H NMR (DMSO- d 6 ): δ 11.2 (s, 2H, Ar-OH), 9.01 (s, 2H, CH=N), 7.37-6.99 (m, Ar-CH). Elemental analysis: calcd for C 14 h 12 N 2 o 2 : C 69.99%, H 5.03%, N 11.66%; Found: C 69.80%, H 5.09%, N 11.59%.
Embodiment 2
[0040] Preparation of salicylaldehyde hydrazide receptor H2
[0041] Under argon protection, add p-nitrosalicylaldehyde and hydrazine hydrate at a molar ratio of 2.5:1 to a round bottom flask, add an appropriate amount of ethanol, stir at room temperature for 12 hours, stop the reaction, filter, wash with ethanol, dry in vacuum, and recrystallize with ethanol Salicylaldehyde hydrazide receptor.
[0042] Salicylaldehyde hydrazide receptor H2: yellow solid, m.p. 251.6-252.1℃; H2 yield: 89.12%; ESI-MS m / e 359.1, 1 H NMR (DMSO- d 6 ): δ 11.42 (s, 2H, Ar-OH), 9.23 (s, 2H, CH=N), 7.52-7.11 (m, Ar-CH). Elemental analysis: calcd for C 16 h 14 N 4 o 6 : C 53.63%, H 3.94%, N 15.64%; Found: C 53.58%, H 3.90, N 15.61%.
Embodiment 3
[0044] Preparation of salicylaldehyde hydrazide receptor H3
[0045] Under nitrogen protection, add 5-methoxy-p-nitrosalicylaldehyde and hydrazine hydrate into a round bottom flask at a molar ratio of 3:1, add an appropriate amount of ethanol, stir at room temperature for 18 hours, stop the reaction, filter, wash with ethanol, and dry in vacuo. Salicylaldehyde hydrazide acceptor was obtained by recrystallization from ethanol.
[0046] Salicylaldehyde hydrazide receptor H3: yellow solid, m.p. 253.5-254.1℃; ESI-MS m / e 391.12, 1 H NMR (CDCl 3 ): δ 11.11 (s, 2H, Ar-OH), 8.96 (s, 2H, CH=N), 7.36-6.33 (m, ArH), 3.83 (s, 6H, CH 3 ).Elemental analysis: calcd for C 18 h 20 N 2 o 4 : C 49.24%, H 3.62%, N 14.35%; Found: C 49.28%, H 3.56%, N 14.32%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com