Metastatic tumor deletion protein inhibitor polypeptide
A technology for protein inhibitors and metastatic tumors, which is applied in the field of preparation and application of recombinant plasmids for protein inhibitors of metastatic tumors, polypeptides, polypeptides and polynucleotides, and can solve the problem that the physiological function of Mtss1 and the influence of its signaling pathway on tumor progression have not been fully elucidated and other problems, to achieve the effect of inhibiting tumor cell internalization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
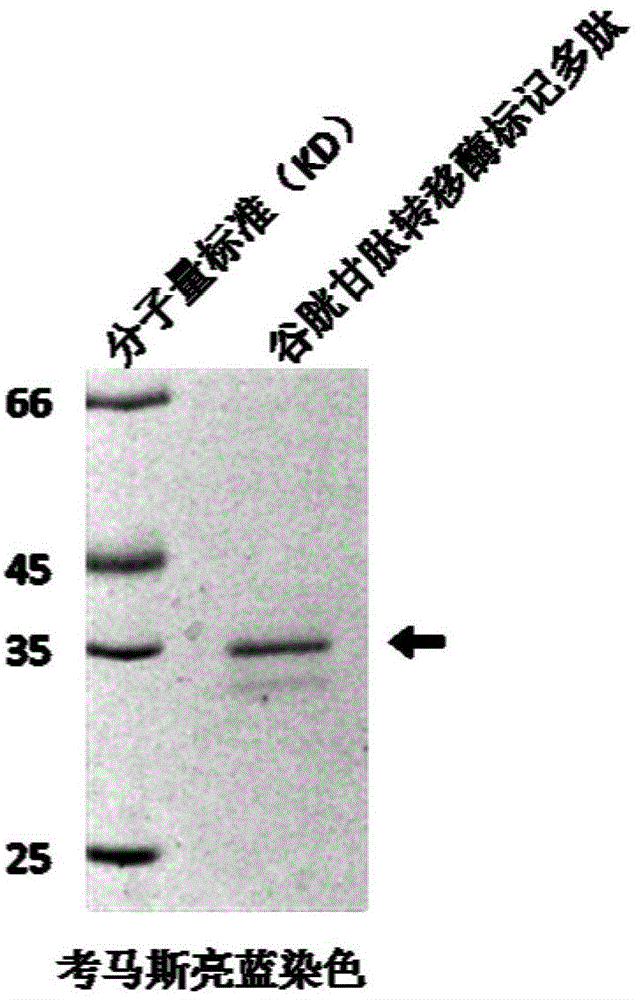
[0028] Example 1 Construction of GST-tagged polypeptide-encoding DNA plasmid.
[0029] The mouse-derived recombinant DNA plasmid pMtss1-Myc-His was used as a template, and the polynucleotides of 5'-CAGTTAGGATCCACCATCAGCGAC-3' and 5'-CAGTTAGTCGACCTAGATGCTCCGGTG-3' sequences were used as primers for PCR amplification. The amplification reaction conditions are: 50μl reaction system, using PfuUltra TM II fusion HS DNA polymerase and its matching 10x buffer, 250 μM of dNTP, 20 ng / μl of template DNA, and 0.5 μM of primer. React 30 cycles on a Mastercycler-type DNA gradient thermal cycler according to the following conditions: 95°C, 20s; 67.4°C, 20s; 72°C, 15s.
[0030] The PCR product was confirmed to have a molecular weight of about 250bp by agarose gel electrophoresis and ultraviolet imaging. It was extracted with a mixture of phenol, chloroform and isoamyl alcohol (25:24:1), and NaAc and Mix with 2 times the amount of absolute ethanol, centrifuge at high speed for 5 minutes, w...
Embodiment 2
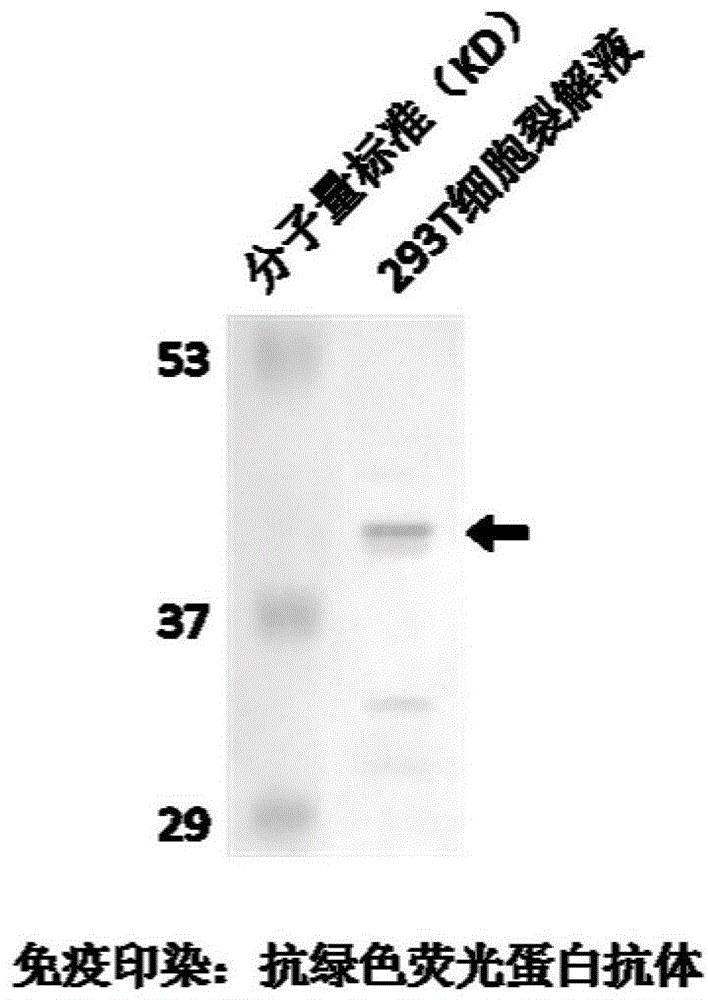
[0033] Example 2 Expression and detection of the inhibitor polypeptide represented by sequence number 2 and its polynucleotide recombinant plasmid in eukaryotic / prokaryotic cells.
[0034] Human embryonic kidney cell 293T cells were cultured using DMEM high-glucose medium containing 10% calf serum BCS, adding penicillin at a final concentration of 100 units / ml and streptomycin at a final concentration of 100 μg / ml. Before DNA transfection, 1.5x10 6 Cells were seeded in 100mm cell culture dishes using normal culture medium without antibiotics at 37°C and 5% CO 2 conditions overnight. Then prepare the transfection mixture: 10 μg green fluorescent protein GFP-tagged inhibitor polypeptide plasmid DNA, 300 μl DMEM, 60 μl SuperFect. After the transfection mixture was incubated at room temperature for 5-10 minutes, add 3ml of normal culture medium and mix well to replace the cell culture medium and culture the cells for 2-3 hours, then replace it with normal culture medium and cult...
Embodiment 3
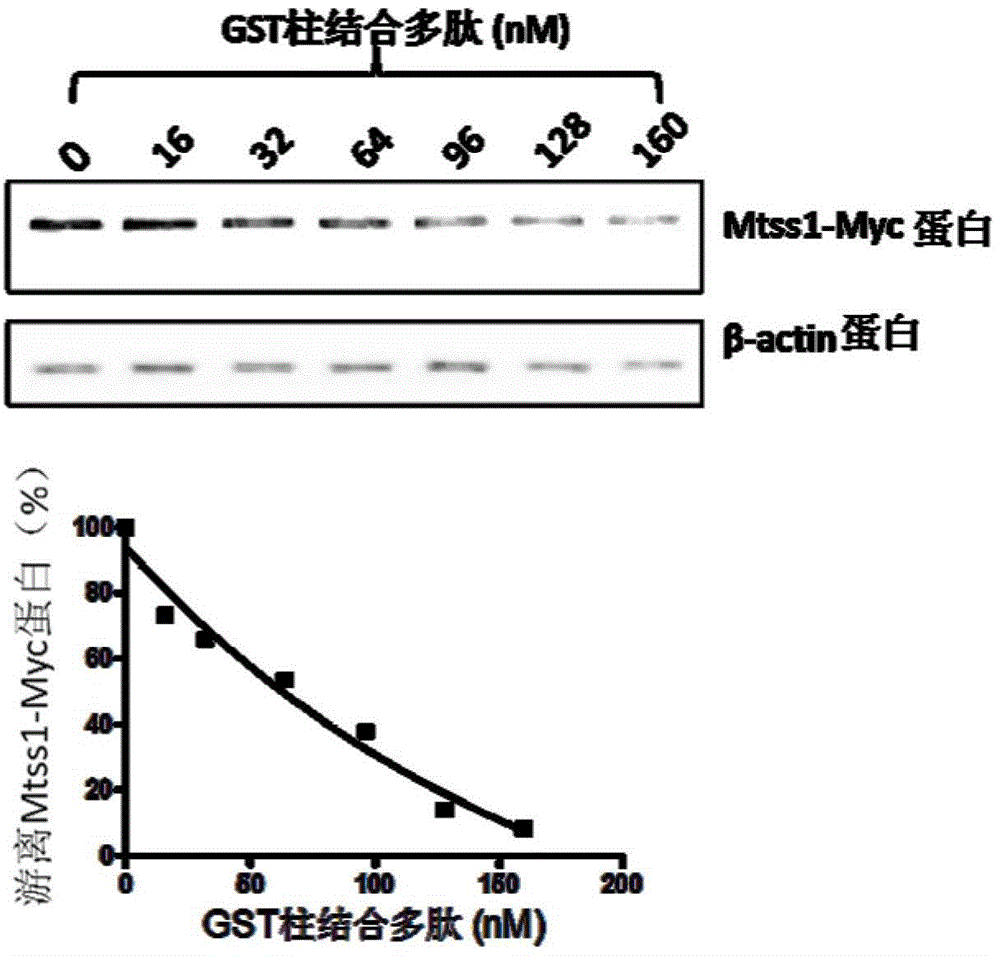
[0037] Example 3 Detection of the binding ability of the inhibitor polypeptide represented by sequence number 2 bound to the GST column to the Mtss1 protein.
[0038] Transfect the pMtss1-Myc-His plasmid into 293T cells, add different concentrations of GST column-bound polypeptides to the expressed cell lysate, incubate at 30 rpm at 4°C for 2 hours, take supernatant samples, and dissolve them with 8% dodecylsulfonate Sodium phosphate (SDS)-polyacrylamide gel electrophoresis for separation. Using the Western Blot method, mouse anti-Myc antibody (9E10) and goat anti-mouse IgG were used to treat respectively, and then developed with chemiluminescence substrate and exposed for imaging to obtain binding ability data, and β-actin was used as an internal reference to determine the binding ability Sample volume (such as image 3 ). The smaller the amount of Mtss1-Myc in the sample, the stronger the binding ability of the GST column to bind the polypeptide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com