Method for regulating expression, quantity and activity of heat shock protein 70 and application thereof
A heat shock protein, active technology, applied in peptide/protein components, medical preparations containing active ingredients, pharmaceutical formulations, etc., can solve the problems of limited stability and solubility, limited clinical application, low bioavailability, etc. Inhibition of solid tumor growth, promotion of activation, and enhancement of sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
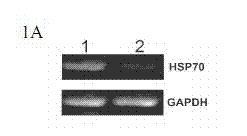
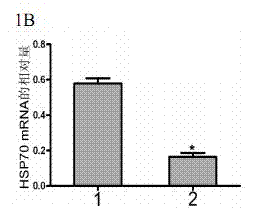
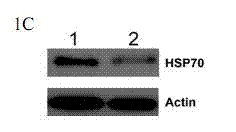
[0072] Using RNA interference technology to down-regulate the expression of HSP70 in tumor cells or tissues, combined with the anti-tumor drug TRAIL to induce tumor cell apoptosis, and treat the mouse A549 solid tumor model as an example:
[0073] 1. Construction of HSP70 interference plasmid:
[0074] Genscript's software siRNA target finder was used to select the appropriate target sequence from the HSP70 sequence, and then Genscript's software siRNA Construct Builder was used to construct a DNA fragment for interference that could be connected to the vector pRNAT-U6.1 / Neo. The DNA fragment includes a sense strand (sense strand, which is the same as the target sequence), a hairpin structure, an antisense strand (antisense strand, which is complementary to the sense strand), and a poly T signal at the 3' end that can terminate transcription; in addition, in order to facilitate It was connected to the vector pRNAT-U6.1 / Neo, and restriction sites for BamH I (G↓GATCC) and Hind I...
Embodiment 2
[0121] Using RNA interference technology to down-regulate the expression of HSP27 in tumor cells or tissues, combined with the anti-tumor drug TRAIL to induce tumor cell apoptosis, and treat the mouse A549 solid tumor model as an example:
[0122] 1. Construction of HSP27 interference plasmid:
[0123] The design and construction method of the HSP27 interference plasmid is the same as that described in Example 1. The fragment for HSP27 RNA interference designed by Gensicript software is as follows: 5'-GGATCCCGTCTCATCGGATTTTGCAGTTGATATCCGCTGCAAAATCCGATGAGACTTTTTTCCAAAAGC-3', and the two fragments synthesized by Shenergy Gambling Biotechnology Co., Ltd. are: 5'-GATCCCGTCTCATCGGATTTTGCAGTTGATATCCGCTGCAAAATCCGATGAGACTTTTTTCCAAA-3' -AGCTTTTGGAAAAAAGTCTCATCGGATTTTGCAGCGGATATCAACTGCAAAATCCGATGAGACGG-3'.
[0124] 2. Detection of the interference effect of HSP27 interference plasmid and its ability to induce A549 cell apoptosis at the cellular level when combined with TRAIL:
[0125]...
Embodiment 3
[0154] Detection of the ability of HSP70 interference combined with TRAIL to induce apoptosis of various non-small cell lung cancer cells NSCLC (A549, SW1573 and H460) at the cellular level (cell apoptosis detected by flow cytometry):
[0155] Transfect the HSP70 interference plasmid into A549, SW1573 and H460 cells with GenEscort Reagent transfection reagent, add TRAIL to induce cell apoptosis 24 hours later, harvest the treated cells after 18 hours, resuspend in 100 μl sample buffer, and keep aside While oscillating, add 1ml of pre-cooled 70% ethanol dropwise, and fix overnight at 4°C. Then centrifuge at 3000rpm for 5 minutes, suck off the supernatant, add 1ml PI staining solution, mix well, place in the dark at room temperature for more than 30 minutes, and detect within 24 hours. Data were collected and analyzed using CellQuest software.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com