Metal carbonyl cobalt cluster and Ti bridged complex, preparation method and application
A technology of cobalt carbonyl and complexes, which is applied in the field of synthesis gas-to-oil catalyst preparation, and can solve problems such as inhomogeneity and random distribution of additive components
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
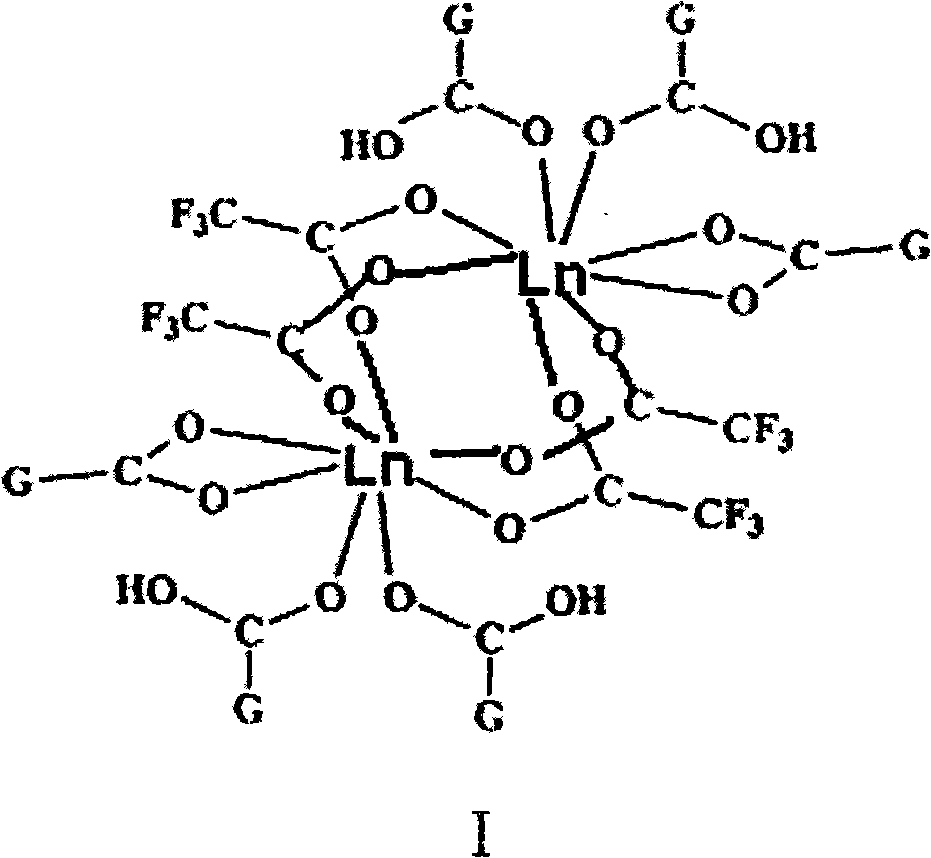
[0049] ln 2 {OOCμ 3 -CCo 3 (CO) 9} 2 {μ-OOCCF 3} 4 {(CO) 9 co 3 mu 3 -CCOOH} 4 Synthesis of (Ln=La, Ce, Eu) (I)
[0050]
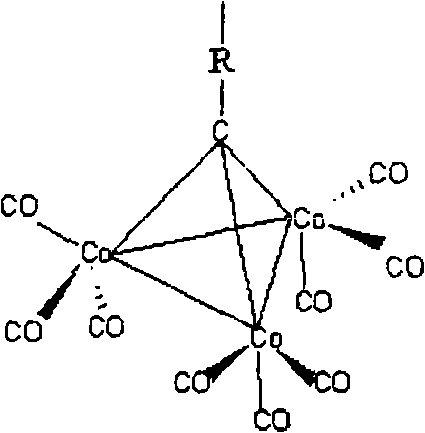
[0051] Will (CO) 9 co 3 CCOOH (0.500g, 1.0mmol) and Ln(OOCCF 3 ) 3 (0.5mmol) dissolved in THF solvent, in 50mL Schlenk bottle N 2 React under protection, stir at 30°C for 1 h to obtain a purple-black solution, and remove the solvent in vacuo. The solid was extracted with hot toluene, and the extract was left at room temperature for 2 days to obtain about 400 mg of black crude crystals, with a yield of about 80% (calculated as Ln).
[0052] Elemental analysis (%, calculated value in brackets), C 74 h 4 co 18 f 12 o 74 Eu 2 : C24.35 (24.22), H 0.43 (0.11), Co 27.96 (28.91), Eu 7.97 (8.28); IR (KBr, cm -1 )2111(m), 2054(vs), 1708(m), 1678(m), 1608(m), 1479(w), 1466(w), 1407(w), 1368(w), 1337(w) , 1265(w), 1205(m), 1155(w), 1062(w), 1028(w), 781(w), 723(w), 528(w), 504(m).
[0053] C 74 h 4 co 18 f 12 o 74 La 2 : C 24.80 (24.40...
Embodiment 2
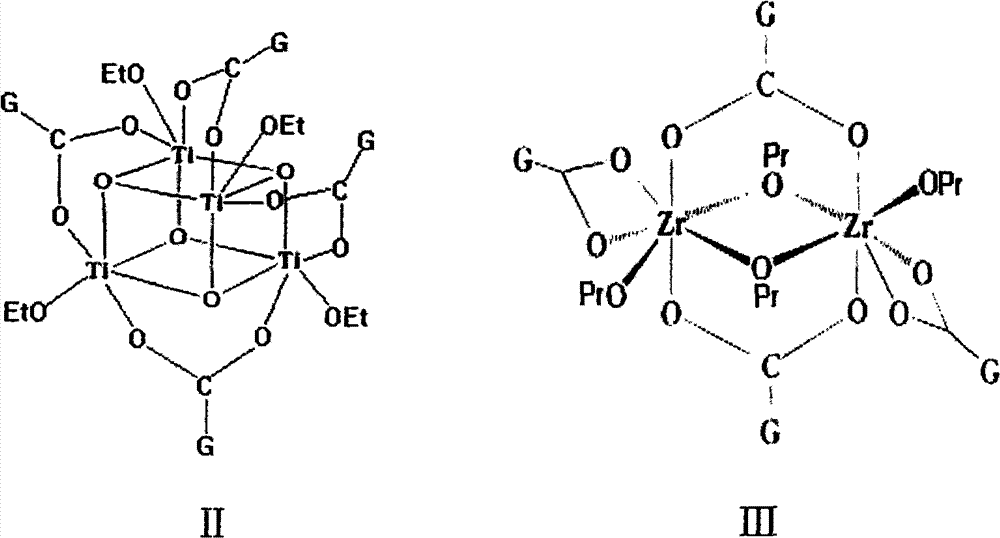
[0056] Ti 4 (μ 3 -O) 4 (OCH 2 CH 3 ) 4 {μ-[(CO) 9 co 3 (μ 3 -CCO 2 )]} 4 Synthesis of (II)
[0057]
[0058] Will (CO) 9 co 3 CCOOH (0.9700g, 2.0mmol) and liquid Ti(OCH 2 CH 3 ) 4 (0.22mL, 1.0mmol) with THF as solvent, in 50mL Schlenk bottle N 2 React under protection, stir at room temperature for 12h to obtain a purple-black solution, and remove the solvent in vacuo. The obtained solid was washed with ethanol until the solution was almost colorless, and then the washed solid was dissolved in 20 ml of dichloromethane, placed at 4° C. for one week, and 350 mg of crystals were collected. Yield 70% (as Ti).
[0059] Elemental analysis (%, calculated value in brackets), C 52 h 20 co 12 Ti 4 o 52 : C: 26.18 (26.29), H: 1.12 (0.85); IR (KBr, cm -1 )2109(m), 2054(vs), 1633(m), 1490(w), 1382(m), 1338(w), 1120(w), 1073(w), 1030(m), 927(w) , 727(w), 618(w), 553(m), 535(m), 502(m).
Embodiment 3
[0061] Yb 4 -O{μ-[Co 3 (CO) 9 mu 3 -CCOO]} 6 Synthesis of (IV)
[0062]
[0063] Will (CO) 9 co 3 CCOOH (0.500g 1.0mmol) and Yb (CF 3 COO) 3 2H 2 O (0.1263g 0.2467mmol) with THF as solvent, in 50mL Schlenk bottle N 2 React under protection, stir at 30°C for 9h to obtain a purple-black solution, and remove the solvent in vacuo. The solid was extracted with hot toluene, and the extract was placed in dichloromethane solvent at room temperature for one week to obtain black crystals.
[0064] Elemental analysis (%, calculated value in brackets): C 67 co 18 o 69 Yb 4 , C: 21.90 (21.85), O: 30.08 (30.12), Co: 28.90 (28.81), Yb: 18.85 (18.79); IR (KBr, cm -1 ): 2109(m), 2011(vs), 1708(m), 1678(m), 1619(m), 1456(w), 1386(w), 1203(m), 1152(w), 1086(w ), 791(w), 752(w), 727(w), 554(w), 502(m).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com