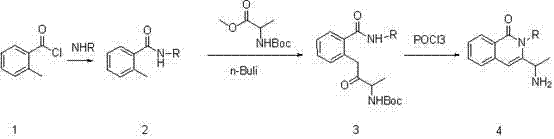
Synthesis of 3-(1-aminoethyl)-2-cyclobutyl-2-hydro-isoquinolin-1-one compound
An aminoethyl and cyclobutyl technology, applied in the field of medicine, can solve the problems of reduced raw material cost, difficulty in purification, and harsh conditions, and achieve the effects of reduced raw material cost, easy purification, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0010] Preparation of compound (2):
[0011] 142 grams (2 moles) of starting material cyclobutylamine was dissolved in 2 methylene chloride, 222 grams (2.2 moles) of triethylamine was added, and 300 grams (2 moles) of o-toluyl chloride was added dropwise under ice-water cooling, After the addition, react at room temperature for 24 hours, add 2 liters of saturated sodium bicarbonate solution, continue to stir for half an hour, separate and extract, wash the organic phase with water, wash with saturated brine, dry over anhydrous sodium sulfate, filter, and spin dry to obtain 344 grams of off-white solid (Yield 91%).
[0012] Preparation of compound (3):
[0013] Dissolve 189 grams (1 mole) of compound (2) in 2 liters of anhydrous tetrahydrofuran, cool it to minus 70 degrees with dry ice / acetone, add 1.2 liters (3 moles) of 2.5 mol / liter butyllithium dropwise, and react at low temperature for half an hour , add 203 g (1 mole) of 2-Boc methyl-alanine in batches, react at room te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com